Elafin and its precursor trappin-2: What is their therapeutic potential for intestinal diseases?
- PMID: 36355635
- PMCID: PMC10098471
- DOI: 10.1111/bph.15985
Elafin and its precursor trappin-2: What is their therapeutic potential for intestinal diseases?
Abstract
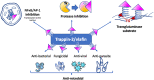
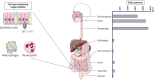
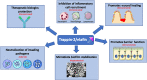
Elafin and its precursor trappin-2 are known for their contribution to the physiological mucosal shield against luminal microbes. Such a contribution seems to be particularly relevant in the gut, where the exposure of host tissues to heavy loads of microbes is constant and contributes to mucosa-associated pathologies. The expression of trappin-2/elafin has been shown to be differentially regulated in diseases associated with gut inflammation. Accumulating evidence has demonstrated the protective effects of trappin-2/elafin in gut intestinal disorders associated with acute or chronic inflammation, or with gluten sensitization disorders. The protective effects of trappin-2/elafin in the gut are discussed in terms of their pleiotropic modes of action: acting as protease inhibitors, transglutaminase substrates, antimicrobial peptides or as a regulator of pro-inflammatory transcription factors. Further, the question of the therapeutic potential of trappin-2/elafin delivery at the intestinal mucosa surface is raised. Whether trappin-2/elafin mucosal delivery should be considered to ensure intestinal tissue repair is also discussed.
Keywords: colitis; elafin; inflammation; inflammatory bowel disease; protease; trappin.
© 2022 The Authors. British Journal of Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Conflict of interest statement
KR is an employee of Nexbiome Therapeutics. NV and PL are inventors on a patent (9688742). All other authors have no conflict of interest.
Figures

Similar articles
-
Secretory leukocyte protease inhibitor (SLPI) is, like its homologue trappin-2 (pre-elafin), a transglutaminase substrate.PLoS One. 2011;6(6):e20976. doi: 10.1371/journal.pone.0020976. Epub 2011 Jun 7. PLoS One. 2011. PMID: 21687692 Free PMC article.
-
Protease inhibitors derived from elafin and SLPI and engineered to have enhanced specificity towards neutrophil serine proteases.Protein Sci. 2009 Mar;18(3):579-94. doi: 10.1002/pro.64. Protein Sci. 2009. PMID: 19241385 Free PMC article.
-
Elafin and its precursor trappin-2 still inhibit neutrophil serine proteinases when they are covalently bound to extracellular matrix proteins by tissue transglutaminase.Biochemistry. 2005 Nov 29;44(47):15610-8. doi: 10.1021/bi051418i. Biochemistry. 2005. PMID: 16300411
-
The WAP protein Trappin-2/Elafin: a handyman in the regulation of inflammatory and immune responses.Int J Biochem Cell Biol. 2012 Aug;44(8):1377-80. doi: 10.1016/j.biocel.2012.05.007. Epub 2012 May 23. Int J Biochem Cell Biol. 2012. PMID: 22634606 Review.
-
SLPI and trappin-2 as therapeutic agents to target airway serine proteases in inflammatory lung diseases: current and future directions.Biochem Soc Trans. 2011 Oct;39(5):1441-6. doi: 10.1042/BST0391441. Biochem Soc Trans. 2011. PMID: 21936830 Review.
Cited by
-
Mucosal expression of PI3, ANXA1, and VDR discriminates Crohn's disease from ulcerative colitis.Sci Rep. 2023 Oct 27;13(1):18421. doi: 10.1038/s41598-023-45569-3. Sci Rep. 2023. PMID: 37891214 Free PMC article.
-
Neutrophil Elastase and Elafin in Inflammatory Bowel Diseases: Urinary Biomarkers Reflecting Intestinal Barrier Dysfunction and Proteolytic Activity.J Clin Med. 2025 Apr 4;14(7):2466. doi: 10.3390/jcm14072466. J Clin Med. 2025. PMID: 40217915 Free PMC article.
-
PI3 expression predicts recurrence after chemotherapy with DNA-damaging drugs in gastric cancer.J Pathol. 2025 Apr;265(4):472-485. doi: 10.1002/path.6400. Epub 2025 Feb 20. J Pathol. 2025. PMID: 39980125 Free PMC article.
-
Neutrophils and NETs in Pathophysiology and Treatment of Inflammatory Bowel Disease.Int J Mol Sci. 2025 Jul 23;26(15):7098. doi: 10.3390/ijms26157098. Int J Mol Sci. 2025. PMID: 40806230 Free PMC article. Review.
-
Expression of Elafin and CD200 as Immune Checkpoint Molecules Involved in Celiac Disease.Int J Mol Sci. 2024 Jan 10;25(2):852. doi: 10.3390/ijms25020852. Int J Mol Sci. 2024. PMID: 38255930 Free PMC article.
References
-
- Alexander, S. P. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Southan, C. , Davies, J. A. , Abbracchio, M. P. , Alexander, W. , Al‐hosaini, K. , Bäck, M. , Barnes, N. M. , Bathgate, R. , … Ye, R. D. (2021). THE CONCISE GUIDE TO PHARMACOLOGY 2021/22: G protein‐coupled receptors. British Journal of Pharmacology, 178(S1), S27–S156. 10.1111/bph.15538 - DOI - PubMed
-
- Alexander, S. P. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Southan, C. , Davies, J. A. , Beuve, A. , Brouckaert, P. , Bryant, C. , Burnett, J. C. , Farndale, R. W. , Friebe, A. , Garthwaite, J. , … Waldman, S. A. (2021a). THE CONCISE GUIDE TO PHARMACOLOGY 2021/22: Catalytic receptors. British Journal of Pharmacology, 178(S1), S264–S312. 10.1111/bph.15541 - DOI - PubMed
-
- Alexander, S. P. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Southan, C. , Davies, J. A. , Boison, D. , Burns, K. E. , Dessauer, C. , Gertsch, J. , Helsby, N. A. , Izzo, A. A. , Koesling, D. , … Wong, S. S. (2021b). THE CONCISE GUIDE TO PHARMACOLOGY 2021/22: Enzymes. British Journal of Pharmacology, 178(S1), S313–S411. 10.1111/bph.15542 - DOI - PubMed
-
- Alexander, S. P. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Southan, C. , Buneman, O. P. , Cidlowski, J. A. , Christopoulos, A. , Davenport, A. P. , Fabbro, D. , Spedding, M. , Striessnig, J. , Davies, J. A. , Ahlers‐Dannen, K. E. , … Zolghadri, Y. (2021). THE CONCISE GUIDE TO PHARMACOLOGY 2021/22: Other protein targets. British Journal of Pharmacology, 178(S1), S1–S26. 10.1111/bph.15537 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

