Pharmacokinetics, pharmacodynamics, safety, and efficacy of crizanlizumab in patients with sickle cell disease
- PMID: 36355805
- PMCID: PMC10027508
- DOI: 10.1182/bloodadvances.2022008209
Pharmacokinetics, pharmacodynamics, safety, and efficacy of crizanlizumab in patients with sickle cell disease
Abstract
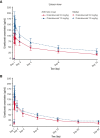
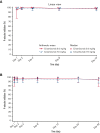
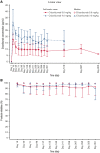
Crizanlizumab is an anti-P-selectin monoclonal antibody indicated to reduce the frequency/prevent recurrence of vaso-occlusive crises (VOCs) in patients with sickle cell disease (SCD) aged ≥16 years. This analysis of an ongoing phase 2, nonrandomized, open-label study reports the pharmacokinetics (PK), pharmacodynamics (PD), safety, and efficacy of crizanlizumab 5.0 mg/kg (N = 45) and 7.5 mg/kg (N = 12) in patients with SCD with a history of VOCs. The median treatment duration was 104.7 and 85.7 weeks in the 5.0 and 7.5 mg/kg groups, respectively. For both doses, serum crizanlizumab concentrations rose to near maximum levels shortly after infusion, and near complete and sustained ex vivo P-selectin inhibition was observed. Grade ≥3 adverse events (AEs) occurred in 48.9% and 33.3% of patients in the 5.0 and 7.5 mg/kg groups, respectively; only 1 event was deemed treatment-related (7.5 mg/kg group). No treatment-related serious AEs occurred. One infusion-related reaction was recorded (5.0 mg/kg, grade 2 "pain during infusion"), which resolved without treatment withdrawal. Infections occurred in 57.8% and 41.7% of patients in the 5.0 and 7.5 mg/kg groups, respectively; none were drug-related. No treatment-related bleeding events were reported. No patients developed immunogenicity. The median (range) absolute reduction from baseline in the annualized rate of VOCs leading to a health care visit was -0.88 (-14.7 to 13.3) and -0.93 (-2.0 to 0.4) in the 5.0 and 7.5 mg/kg groups, respectively. Results here demonstrate the PK/PD properties of crizanlizumab in patients with SCD and the potential sustained efficacy and long-term safety of the drug after >12 months' treatment. This trial was registered at www.clinicaltrials.gov as #NCT03264989.
Trial registration: ClinicalTrials.gov NCT03264989 NCT01895361.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: J.K. reports consultancy for Novartis, Forma Therapeutics, Graphite Bio, and Fulcrum Therapeutics and participation in an advisory board meeting for Novartis, Forma Therapeutics, Fulcrum Therapeutics, Novo Nordisk, and Beam Therapeutics. R.C.B. reports consultancy for Global Blood Therapeutics, Imara, Novartis, and Novo Nordisk and research funding for Global Blood Therapeutics, Imara, Novartis, Forma Therapeutics, and Pfizer. C.N. reports participation in an advisory board meeting for Global Blood Therapeutics and Forma Therapeutics and research funding for Global Blood Therapeutics and Novartis. A.K. reports consultancy for Novartis and Guidepoint; research funding for Global Blood Therapeutics and Forma Therapeutics; participation in a speakers bureau for Global Blood Therapeutics; membership on a scientific committee for bluebird bio and Graphite Bio; participation in an advisory board meeting for Novartis; and participation in an adjudication committee for Vertex. D.M. reports consultancy for Novartis, Global Blood Therapeutics, bluebird bio, and Forma Therapeutics; honoraria from the Research Triangle Institute and the Cure Sickle Cell Initiative, each on behalf of the National Heart, Lung and Blood Institute, National Institutes of Health; and research funding from Grifols. N.S. reports consultancy for Novartis and Global Blood Therapeutics; research funding from Novartis; participation in an advisory board meeting for Global Blood Therapeutics and Forma Therapeutics; and participation in a speakers bureau for Novartis and Global Blood Therapeutics. C.T., G.S.-O., and U.A. are employees of Novartis Pharma AG. S.B. was an employee of IQVIA at the time of writing. The remaining authors declare no competing financial interests.
The current affiliation for R.C.B. is Cytel, London, United Kingdom.
The current affiliation for S.B. is Global Blood Therapeutics, South San Francisco, CA.
Figures


References
-
- Kato GJ, Piel FB, Reid CD, et al. Sickle cell disease. Nat Rev Dis Prim. 2018;4:18010. - PubMed

