Optimal timing of steroid initiation in response to CTLA-4 antibody in metastatic cancer: A mathematical model
- PMID: 36355837
- PMCID: PMC9648769
- DOI: 10.1371/journal.pone.0277248
Optimal timing of steroid initiation in response to CTLA-4 antibody in metastatic cancer: A mathematical model
Abstract
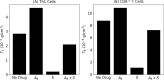
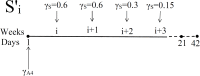
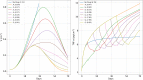
Immune checkpoint inhibitors, introduced in recent years, have revolutionized the treatment of many cancers. However, the toxicity associated with this therapy may cause severe adverse events. In the case of advanced lung cancer or metastatic melanoma, a significant number (10%) of patients treated with CTLA-4 inhibitor incur damage to the pituitary gland. In order to reduce the risk of hypophysitis and other severe adverse events, steroids may be combined with CTLA-4 inhibitor; they reduce toxicity, but they also diminish the anti-cancer effect of the immunotherapy. This trade-off between tumor reduction and the risk of severe adverse events poses the following question: What is the optimal time to initiate treatment with steroid. We address this question with a mathematical model from which we can also evaluate the comparative benefits of each schedule of steroid administration. In particular, we conclude that treatment with steroid should not begin too early, but also not very late, after immunotherapy began; more precisely, it should start as soon as tumor volume, under the effect of CTLA-4 inhibitor alone, begins to decrease. We can also compare the benefits of short term treatment of steroid at high doses to a longer term treatment with lower doses.
Copyright: © 2022 Siewe, Friedman. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




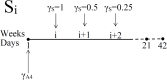



References
-
- Fucà G, Galli G, Poggi M, Lo Russo G, Proto C, Imbimbo M, et al. Modulation of peripheral blood immune cells by early use of steroids and its association with clinical outcomes in patients with metastatic non-small cell lung cancer treated with immune checkpoint inhibitors. ESMO Open. 2019;4(1):e000457. doi: 10.1136/esmoopen-2018-000457 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

