Determinants of hydroxyurea use among doctors, nurses and sickle cell disease patients in Nigeria
- PMID: 36355850
- PMCID: PMC9682971
- DOI: 10.1371/journal.pone.0276639
Determinants of hydroxyurea use among doctors, nurses and sickle cell disease patients in Nigeria
Abstract
Background: Hydroxyurea (HU) is an evidence-based therapy that is currently the most effective drug for sickle cell disease (SCD). HU is widely used in high-income countries with consequent reduction of morbidity and mortality. In Nigeria, HU is prescribed by physicians while nurses are mainly involved in counseling the patients to ensure adherence. The extent of utilization and the determinant factors have not been sufficiently evaluated in Nigeria.
Objective: To assess the frequency of use of HU and factors affecting utilization among healthcare providers, patients, and caregivers for SCD.
Methods: A questionnaire was administered online and in- person to assess the frequency of HU use and the factors that promote and limit its use. The data were analyzed by descriptive statistics using IBM SPSS software version 23 and the result was presented in frequency tables and percentages.
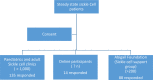
Result: A total of 137 physicians, 137 nurses, and 237 patients/caregivers responded to the survey. The rate of prescription of HU by doctors in the past 6 months was 64 (46.7%), 43 (31.4%) nurses provided counseling and 36 (15.6%) patients were on HU. Among doctors, adequate knowledge (91.3%), clinical benefits and safety (94.8%), and inclusion of HU in management guidelines (86.9%) were motivators for prescribing it while inadequate knowledge (60.9%) and unawareness of treatment guidelines (68.6%) constituted barriers. Among nurses, reduction of crisis (91.6%) and safety (64.8%) were the major motivators while barriers were high cost (79.1%) and intensive monitoring (63.1%) of HU treatment. Among the patients, the major motivator was the reduction of crises (80.3%) while poor knowledge (93.2%), high cost of the drug (92.2%) while monitoring (91.2%), non-availability (87.7%) and side effects (83.9%) were the major barriers for the utilization of HU.
Conclusion: HU prescription and utilization are still poor among healthcare providers and patients. Inadequate knowledge, non-availability and high cost of HU as well as unawareness of treatment guidelines constitute major barriers to prescription and utilization.
Copyright: This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Provider prescription of hydroxyurea in youth and adults with sickle cell disease: A review of prescription barriers and facilitators.Br J Haematol. 2023 Dec;203(5):712-721. doi: 10.1111/bjh.19099. Epub 2023 Sep 10. Br J Haematol. 2023. PMID: 37691131 Free PMC article. Review.
-
Barriers to the use of hydroxyurea in the management of sickle cell disease in Nigeria.Hemoglobin. 2019 May;43(3):188-192. doi: 10.1080/03630269.2019.1649278. Epub 2019 Aug 29. Hemoglobin. 2019. PMID: 31462098
-
Understanding patient-related barriers to hydroxyurea use among adolescent and adult patients with sickle cell disease in Mulago and Kiruddu hospitals, Uganda, a qualitative study.BMC Health Serv Res. 2024 May 27;24(1):666. doi: 10.1186/s12913-024-11125-6. BMC Health Serv Res. 2024. PMID: 38802815 Free PMC article.
-
Perception to hydroxyurea therapy in patients with sickle cell disease: Report from 3 centers.Ann Afr Med. 2021 Apr-Jun;20(2):127-131. doi: 10.4103/aam.aam_36_20. Ann Afr Med. 2021. PMID: 34213480 Free PMC article.
-
Hydroxyurea for the treatment of sickle cell disease.Evid Rep Technol Assess (Full Rep). 2008 Mar;(165):1-95. Evid Rep Technol Assess (Full Rep). 2008. PMID: 18457478 Free PMC article. Review.
Cited by
-
Hydroxyurea therapy for neurological and cognitive protection in pediatric sickle cell anemia in Uganda (BRAIN SAFE II): Protocol for a single-arm open label trial.Contemp Clin Trials Commun. 2024 Nov 28;42:101404. doi: 10.1016/j.conctc.2024.101404. eCollection 2024 Dec. Contemp Clin Trials Commun. 2024. PMID: 39717517 Free PMC article.
-
Hydroxyurea Therapy for Neurological and Cognitive Protection in Pediatric Sickle Cell Anemia in Uganda (BRAIN SAFE II): Protocol for a single-arm open label trial.medRxiv [Preprint]. 2024 Jan 13:2024.01.12.24301208. doi: 10.1101/2024.01.12.24301208. medRxiv. 2024. Update in: Contemp Clin Trials Commun. 2024 Nov 28;42:101404. doi: 10.1016/j.conctc.2024.101404. PMID: 38260320 Free PMC article. Updated. Preprint.
-
Provider prescription of hydroxyurea in youth and adults with sickle cell disease: A review of prescription barriers and facilitators.Br J Haematol. 2023 Dec;203(5):712-721. doi: 10.1111/bjh.19099. Epub 2023 Sep 10. Br J Haematol. 2023. PMID: 37691131 Free PMC article. Review.
References
-
- Ernest B. The Sickle Cell Disease and Related Disorders. In: Williams Haematology. McGraw Hill Medical Publishing Division. New York.6th Ed; 2000: 581–606.
-
- Nnodu OE, Oron AP, Sopekan A, Akaba GO, Piel FB, Chao DL. Child mortality from sickle cell disease in Nigeria: a model-estimated, population-level analysis of data from the 2018 Demographic and Health Survey. Lancet Haematol 2021; 8(10): e723–e31. doi: 10.1016/S2352-3026(21)00216-7 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous