Recombinant Vaccinia Virus Expressing Plasmodium berghei Apical Membrane Antigen 1 or Microneme Protein Enhances Protection against P. berghei Infection in Mice
- PMID: 36355892
- PMCID: PMC9698705
- DOI: 10.3390/tropicalmed7110350
Recombinant Vaccinia Virus Expressing Plasmodium berghei Apical Membrane Antigen 1 or Microneme Protein Enhances Protection against P. berghei Infection in Mice
Abstract
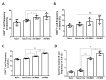
Recombinant vaccinia viruses (rVV) are effective antigen delivery vectors and are researched widely as vaccine platforms against numerous diseases. Apical membrane antigen 1 (AMA1) is one of the candidate antigens for malaria vaccines but rising concerns regarding its genetic diversity and polymorphism have necessitated the need to search for an alternative antigen. Here, we compare the efficacies of the rVV vaccines expressing either AMA1 or microneme protein (MIC) of Plasmodium berghei in mice. Mice (BALB/c) were immunized with either rVV-AMA1 or rVV-MIC and subsequently challenge-infected with P. berghei. Compared to the control group, both antigens elicited elevated levels of parasite-specific antibody responses. Immunization with either one of the two vaccines induced high levels of T cells and germinal center B cell responses. Interestingly, rVV-MIC immunization elicited higher levels of cellular immune response compared to rVV-AMA1 immunization, and significantly reduced pro-inflammatory cytokine productions were observed from the former vaccine. While differences in parasitemia and bodyweight changes were negligible between rVV-AMA1 and rVV-MIC immunization groups, prolonged survival was observed for the latter of the two. Based on these results, our findings suggest that the rVV expressing the P. berghei MIC could be a vaccine-candidate antigen.
Keywords: Plasmodium berghei; apical membrane antigen 1; microneme protein; recombinant vaccinia virus (rVV); vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- WHO . World Malaria Report 2021. World Health Organization; Geneva, Switzerland: 2021.
-
- de Barra E., Hodgson S.H., Ewer K.J., Bliss C.M., Hennigan K., Collins A., Berrie E., Lawrie A.M., Gilbert S.C., Nicosia A., et al. A phase Ia study to assess the safety and immunogenicity of new malaria vaccine candidates ChAd63 CS administered alone and with MVA CS. PLoS ONE. 2014;9:e115161. doi: 10.1371/journal.pone.0115161. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

