Influence of pH on the Kinetics and Products of Photocatalytic Degradation of Sulfonamides in Aqueous Solutions
- PMID: 36355946
- PMCID: PMC9695452
- DOI: 10.3390/toxics10110655
Influence of pH on the Kinetics and Products of Photocatalytic Degradation of Sulfonamides in Aqueous Solutions
Abstract
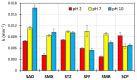
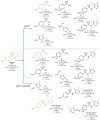
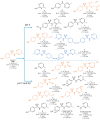
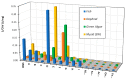
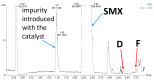
The aims of the study were to determine the kinetics of the photocatalytic degradation of six sulfonamides in the presence of TiO2-P25 in acidic, neutral, and alkaline solutions and to identify the structures of the stable products. It was stated that the pH of the solution significantly affected the photocatalytic degradation rate of sulfonamides in acidic and alkaline environments, and the effect likely depended on the susceptibility of sulfonamides to attack by hydroxyl radicals. In the post-reaction mixture, we identified the compounds resulting from the substitution of the aromatic rings with a hydroxyl group; the amide hydrolysis products; the hydroxylamine-, azo, and nitro derivatives; and the compounds formed via the elimination of the sulfone group. Moreover, previously unknown azo compounds were detected. Some degradation products of sulfonamides may exhibit marked bacteriostatic activity and high phytotoxicity. The azo and nitro compounds formed in an acidic environment may be potentially more toxic to aquatic ecosystems than the initial compounds.
Keywords: degradation products; ecotoxicity; mechanism; photocatalysis; sulfonamides.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

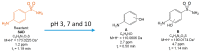



References
-
- European Medicines Agency Sales of Veterinary Antimicrobial Agents in 31 European Countries in 2019 and 2020. [(accessed on 1 August 2022)]. Available online: https://www.ema.europa.eu/en/documents/report/sales-veterinary-antimicro....
-
- FDA Antimicrobials Sold or Distributed for Use in Food-Producing Animals. [(accessed on 1 August 2022)]; Available online: https://www.fda.gov/animal-veterinary/cvm-updates/fda-releases-annual-su....
-
- Winfieldunited United Asulam 3.3. [(accessed on 1 August 2022)]. Available online: https://www.winfieldunited.com/products/herbicides/asulam33/84.
Grants and funding
LinkOut - more resources
Full Text Sources

