Flavor Classification/Categorization and Differential Toxicity of Oral Nicotine Pouches (ONPs) in Oral Gingival Epithelial Cells and Bronchial Epithelial Cells
- PMID: 36355951
- PMCID: PMC9696007
- DOI: 10.3390/toxics10110660
Flavor Classification/Categorization and Differential Toxicity of Oral Nicotine Pouches (ONPs) in Oral Gingival Epithelial Cells and Bronchial Epithelial Cells
Abstract
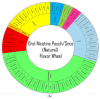
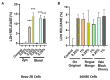
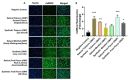
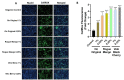
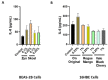
Oral nicotine pouches (ONPs) are a modern form of smokeless tobacco products sold by several brands in the U.S., which comprise a significant portion of non-combustible nicotine-containing product (NCNP) sales to date. ONPs are available in various flavors and may contain either tobacco-derived nicotine (TDN) or tobacco-free nicotine (TFN). The growth in popularity of these products has raised concerns that flavored ONPs may cause adverse oral health effects and promote systemic toxic effects due to nicotine and other ONP by-products being absorbed into the circulatory system through oral mucosa. We hypothesized that flavored ONPs are unsafe and likely to cause oral and pulmonary inflammation in oral and respiratory epithelial cells. Before analyzing the effects of ONPs, we first classified ONPs sold in the U.S. based on their flavor and the flavor category to which they belonged using a wheel diagram. Human gingival epithelial cells (HGEP) were treated with flavored ONP extracts of tobacco (original, smooth), menthol (wintergreen and cool cider), and fruit flavor (americana and citrus), each from the TDN and TFN groups. The levels of ONP-induced inflammatory cytokine release (TNF-α, IL-6, and IL-8) by ELISA, cellular reactive oxygen species (ROS) production by CellRox Green, and cytotoxicity by lactate dehydrogenase (LDH) release assay in HGEP cells were assessed. Flavored ONP extracts elicited differential toxicities in a dose- and extract-dependent manner in HGEP cells 24 h post-treatment. Both fruit TDN and TFN extracts resulted in the greatest cytotoxicity. Tobacco- and fruit-flavored, but not menthol-flavored, ONPs resulted in increased ROS production 4 h post-treatment. Flavored ONPs led to differential cytokine release (TNF-α, IL-6, and IL-8) which varied by flavor (menthol, tobacco, or fruit) and nicotine (TDN vs. TFN) 24 h post-treatment. Menthol-flavored ONPs led to the most significant TNF-α release; fruit TFN resulted in the most significant IL-6 release; and fruit TDN and tobacco TFN led to the highest release of IL-8. Subsequently, human bronchial epithelial cells (16-HBE and BEAS-2B) were also treated with flavored ONP extracts, and similar assays were evaluated. Here, the lowest concentration treatments displayed increased cytotoxicity. The most striking response was observed among cells treated with spearmint and tobacco flavored ONPs. Our data suggest that flavored ONPs are unsafe and likely to cause systemic and local toxicological responses during chronic usage.
Keywords: cytotoxicity; inflammation; oral nicotine pouches; periodontal problems; pulmonary health.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Classification, Perception, and Toxicity of Emerging Flavored Oral Nicotine Pouches.Int J Environ Res Public Health. 2023 Mar 3;20(5):4526. doi: 10.3390/ijerph20054526. Int J Environ Res Public Health. 2023. PMID: 36901533 Free PMC article. Review.
-
Synthetic Cooling Agent in Oral Nicotine Pouch Products Marketed as "Flavor-Ban Approved".bioRxiv [Preprint]. 2023 Feb 24:2023.02.23.529797. doi: 10.1101/2023.02.23.529797. bioRxiv. 2023. Update in: Tob Control. 2025 Jan 2;34(1):106-110. doi: 10.1136/tc-2023-058035. PMID: 36865160 Free PMC article. Updated. Preprint.
-
Comparison of Nicotine and Selected Flavorings Content Between Tobacco-Free and Tobacco-Containing Oral Pouches.Nicotine Tob Res. 2025 May 19:ntaf105. doi: 10.1093/ntr/ntaf105. Online ahead of print. Nicotine Tob Res. 2025. PMID: 40383714
-
Evaluating the Role of Nicotine Stereoisomer on Nicotine Pouch Abuse Liability: A Randomized Crossover Trial.Nicotine Tob Res. 2025 Mar 24;27(4):658-665. doi: 10.1093/ntr/ntae079. Nicotine Tob Res. 2025. PMID: 38713545 Free PMC article. Clinical Trial.
-
The Potential Impact of Oral Nicotine Pouches on Public Health: A Scoping Review.Nicotine Tob Res. 2025 Mar 24;27(4):598-610. doi: 10.1093/ntr/ntae131. Nicotine Tob Res. 2025. PMID: 38880491 Free PMC article.
Cited by
-
Classification, Perception, and Toxicity of Emerging Flavored Oral Nicotine Pouches.Int J Environ Res Public Health. 2023 Mar 3;20(5):4526. doi: 10.3390/ijerph20054526. Int J Environ Res Public Health. 2023. PMID: 36901533 Free PMC article. Review.
-
What is the impact of nicotine pouches on oral health: a systematic review.BMC Oral Health. 2024 Aug 3;24(1):889. doi: 10.1186/s12903-024-04598-8. BMC Oral Health. 2024. PMID: 39097712 Free PMC article.
-
Oral nicotine pouches with an aftertaste? Part 2: in vitro toxicity in human gingival fibroblasts.Arch Toxicol. 2023 Sep;97(9):2343-2356. doi: 10.1007/s00204-023-03554-9. Epub 2023 Jul 23. Arch Toxicol. 2023. PMID: 37482550 Free PMC article.
-
Comparative Cytotoxicity of Menthol and Eucalyptol: An In Vitro Study on Human Gingival Fibroblasts.Pharmaceutics. 2024 Apr 9;16(4):521. doi: 10.3390/pharmaceutics16040521. Pharmaceutics. 2024. PMID: 38675182 Free PMC article.
-
Small pouches, but high nicotine doses-nicotine delivery and acute effects after use of tobacco-free nicotine pouches.Front Pharmacol. 2024 May 22;15:1392027. doi: 10.3389/fphar.2024.1392027. eCollection 2024. Front Pharmacol. 2024. PMID: 38841367 Free PMC article.
References
-
- Gentzke A.S., Wang T.W., Cornelius M., Park-Lee E., Ren C., Sawdey M.D., Cullen K.A., Loretan C., Jamal A., Homa D.M. Tobacco Product Use and Associated Factors Among Middle and High School Students—National Youth Tobacco Survey, United States, 2021. MMWR Surveill. Summ. 2021;71:1–29. doi: 10.15585/mmwr.ss7105a1. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

