Long-Term Consumption of Food-Derived Chlorogenic Acid Protects Mice against Acetaminophen-Induced Hepatotoxicity via Promoting PINK1-Dependent Mitophagy and Inhibiting Apoptosis
- PMID: 36355956
- PMCID: PMC9693533
- DOI: 10.3390/toxics10110665
Long-Term Consumption of Food-Derived Chlorogenic Acid Protects Mice against Acetaminophen-Induced Hepatotoxicity via Promoting PINK1-Dependent Mitophagy and Inhibiting Apoptosis
Abstract
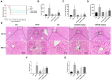
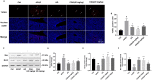
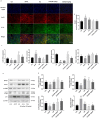
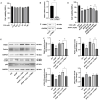
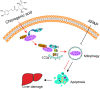
Hepatotoxicity brought on by acetaminophen (APAP) is significantly impacted by mitochondrial dysfunction. Mitophagy, particularly PINK1-mediated mitophagy, maintains the stability of cell function by eliminating damaged mitochondria. One of the most prevalent dietary polyphenols, chlorogenic acid (CGA), has been shown to have hepatoprotective properties. It is yet unknown, nevertheless, whether its defense against hepatocyte apoptosis involves triggering PINK1-mediated mitophagy. In vitro and in vivo models of APAP-induced hepatotoxicity were established to observe CGA's effect and mechanism in preventing hepatotoxicity in the present study. Serum aminotransferase levels, mouse liver histology, and the survival rate of HepG2 cells and mice were also assessed. The outcomes showed that CGA could reduce the activities of serum enzymes such as alanine transaminase (ALT), aspartate transaminase (AST), and lactate dehydrogenase (LDH), and alleviate liver injury in mice. It could also significantly increase the cell viability of HepG2 cells and the 24-h survival rate of mice. TUNEL labeling and Western blotting were used to identify the hepatocyte apoptosis level. According to data, CGA could significantly reduce liver cell apoptosis in vivo. Additionally, Tom20 and LC3II colocalization in mitochondria may be facilitated by CGA. CGA considerably increased the levels of genes and proteins associated with mitophagy (PINK1, Parkin, LC3II/LC3I), while considerably decreasing the levels of p62 and Tom20, suggesting that it might activate PINK1/Parkin-mediated mitophagy in APAP-induced liver damage. Additionally, the protection of CGA was reduced when PINK1 was knocked down by siPINK1 in HepG2 cells, and it did not upregulate mitophagy-related proteins (PINK1, Parkin, LC3II/LC3I). In conclusion, our findings revealed that long-term consumption of food-derived CGA could prevent APAP hepatotoxicity via increasing PINK1-dependent mitophagy and inhibiting hepatocyte apoptosis.
Keywords: APAP-induced liver injury; PINK1/Parkin pathway; apoptosis; chlorogenic acid; mitophagy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Double deletion of PINK1 and Parkin impairs hepatic mitophagy and exacerbates acetaminophen-induced liver injury in mice.Redox Biol. 2019 Apr;22:101148. doi: 10.1016/j.redox.2019.101148. Epub 2019 Feb 20. Redox Biol. 2019. PMID: 30818124 Free PMC article.
-
Matrine promotes liver cancer cell apoptosis by inhibiting mitophagy and PINK1/Parkin pathways.Cell Stress Chaperones. 2018 Nov;23(6):1295-1309. doi: 10.1007/s12192-018-0937-7. Epub 2018 Sep 12. Cell Stress Chaperones. 2018. Retraction in: Cell Stress Chaperones. 2021 Nov;26(6):1009. doi: 10.1007/s12192-021-01225-1. PMID: 30209783 Free PMC article. Retracted.
-
Mitophagy protects against acetaminophen-induced acute liver injury in mice through inhibiting NLRP3 inflammasome activation.Biochem Pharmacol. 2019 Nov;169:113643. doi: 10.1016/j.bcp.2019.113643. Epub 2019 Sep 19. Biochem Pharmacol. 2019. PMID: 31542387
-
[Discovery of miRNA and target signal molecules involved in inhibition of chlorogenic acid on N-acetyl-p-aminophenol-induced hepatotoxicity based on microRNA array].Zhongguo Zhong Yao Za Zhi. 2023 Feb;48(4):1014-1022. doi: 10.19540/j.cnki.cjcmm.20221017.401. Zhongguo Zhong Yao Za Zhi. 2023. PMID: 36872272 Chinese.
-
Targeting Pink1-Parkin-mediated mitophagy for treating liver injury.Pharmacol Res. 2015 Dec;102:264-9. doi: 10.1016/j.phrs.2015.09.020. Epub 2015 Oct 24. Pharmacol Res. 2015. PMID: 26655101 Free PMC article. Review.
Cited by
-
Functional foods and bioactive compounds: a comprehensive review on their role in mitigating drug-induced liver injury.Front Nutr. 2025 May 21;11:1499697. doi: 10.3389/fnut.2024.1499697. eCollection 2024. Front Nutr. 2025. PMID: 40469945 Free PMC article. Review.
-
Chlorogenic Acid: A Systematic Review on the Biological Functions, Mechanistic Actions, and Therapeutic Potentials.Nutrients. 2024 Mar 23;16(7):924. doi: 10.3390/nu16070924. Nutrients. 2024. PMID: 38612964 Free PMC article.
-
EGR1 is crucial for the chlorogenic acid-provided promotion on liver regeneration and repair after APAP-induced liver injury.Cell Biol Toxicol. 2023 Dec;39(6):2685-2707. doi: 10.1007/s10565-023-09795-9. Epub 2023 Feb 21. Cell Biol Toxicol. 2023. PMID: 36809385
-
Targeting mitophagy for neurological disorders treatment: advances in drugs and non-drug approaches.Naunyn Schmiedebergs Arch Pharmacol. 2023 Dec;396(12):3503-3528. doi: 10.1007/s00210-023-02636-w. Epub 2023 Aug 3. Naunyn Schmiedebergs Arch Pharmacol. 2023. PMID: 37535076 Review.
-
Acetaminophen-induced liver injury: Molecular mechanism and treatments from natural products.Front Pharmacol. 2023 Mar 27;14:1122632. doi: 10.3389/fphar.2023.1122632. eCollection 2023. Front Pharmacol. 2023. PMID: 37050900 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

