Role of PI3K/Akt-Mediated Nrf2/HO-1 Signaling Pathway in Resveratrol Alleviation of Zearalenone-Induced Oxidative Stress and Apoptosis in TM4 Cells
- PMID: 36355983
- PMCID: PMC9694162
- DOI: 10.3390/toxins14110733
Role of PI3K/Akt-Mediated Nrf2/HO-1 Signaling Pathway in Resveratrol Alleviation of Zearalenone-Induced Oxidative Stress and Apoptosis in TM4 Cells
Abstract
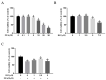
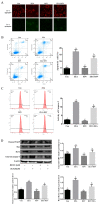
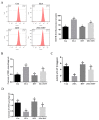
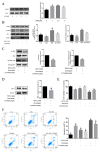
Zearalenone (ZEA) is a common mycotoxin that induces oxidative stress (OS) and affects the male reproductive system in animals. Resveratrol (RSV) has good antioxidant activity and can activate nuclear factor erythroid 2-related factor (Nrf2) to protect cells through the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) signaling pathway. The objective of this study was to investigate the protective effect and the mechanism of RSV on OS and apoptosis in TM4 cells induced by ZEA. Prior to being exposed to ZEA, TM4 cells were pretreated with RSV or the PI3K/Akt inhibitor LY294002. Cell viability was measured by Cell Counting Kit-8 (CCK-8) assays. Flow cytometry was used to determine the level of apoptosis and intracellular reactive oxygen species (ROS). The expression of poly ADP-ribose polymerase (PARP), caspase-3, BCL2-associated X (Bax)/B-cell lymphoma-2 (Bcl-2), and PI3K/Akt-mediated Nrf2/heme oxygenase 1 (HO-1) signaling pathway-related proteins was evaluated by Western blotting. Nrf2 siRNA transfection and LY294002 treatment were used to investigate the role of the Nrf2/HO-1 and PI3K/Akt signaling pathways in RSV alleviation of ZEA-induced OS. The results showed that pretreatment with RSV significantly reduced the expression of apoptosis-related proteins and increased cell viability. Catalase (CAT) activity and glutathione (GSH) levels were also increased, whereas malondialdehyde (MDA) and ROS levels decreased (p < 0.05). RSV also upregulated Akt phosphorylation, Nrf2 nuclear translocation, and HO-1 expression under conditions of OS (p < 0.05). Transfection with Nrf2 siRNA abolished the protective effects of RSV against ZEA-induced cytotoxicity (p < 0.05), ROS accumulation (p < 0.05), and apoptosis (p < 0.05). LY294002 completely blocked the RSV-mediated increase in Nrf2 nuclear translocation (p < 0.05), HO-1 expression (p < 0.05), and cytoprotective activity (p < 0.05). Collectively, the above findings indicate that RSV can protect against ZEA-induced OS and apoptosis in TM4 cells by PI3K/Akt-mediated activation of the Nrf2/HO-1 signaling pathway.
Keywords: Nrf2/HO-1 signaling pathway; PI3K/Akt signaling pathway; ROS; TM4 cells; cytotoxicity; oxidative damage; resveratrol; zearalenone.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Cao L., Zhao J., Ma L., Chen J., Xu J., Rahman S.U., Feng S., Li Y., Wu J., Wang X. Lycopene attenuates zearalenone-induced oxidative damage of piglet sertoli cells through the nuclear factor erythroid-2 related factor 2 signaling pathway. Ecotoxicol. Environ. Saf. 2021;225:112737. doi: 10.1016/j.ecoenv.2021.112737. - DOI - PubMed
-
- Huang H., Lv L., Wang D., Guo B., Lv J., Luo L., Wen B., Kang Y. Biochemical and molecular responses of maize (Zea mays L.) to 1,2-dibromo-4-(1,2 dibromoethyl) cyclohexane (TBECH) diastereomers: Oxidative stress, DNA damage, antioxidant enzyme gene expression and diversity of root exudates. Sci. Total Environ. 2021;753:141872. doi: 10.1016/j.scitotenv.2020.141872. - DOI - PubMed
-
- Feng N., Wang B., Cai P., Zheng W., Zou H., Gu J., Yuan Y., Liu X., Liu Z., Bian J. ZEA-induced autophagy in TM4 cells was mediated by the release of Ca2+ activates CaMKKβ-AMPK signaling pathway in the endoplasmic reticulum. Toxicol. Lett. 2020;323:1–9. doi: 10.1016/j.toxlet.2020.01.010. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

