AbobotulinumtoxinA Doses in Upper and Lower Limb Spasticity: A Systematic Literature Review
- PMID: 36355984
- PMCID: PMC9698883
- DOI: 10.3390/toxins14110734
AbobotulinumtoxinA Doses in Upper and Lower Limb Spasticity: A Systematic Literature Review
Abstract
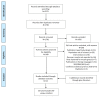
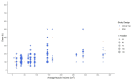
Disabling limb spasticity can result from stroke, traumatic brain injury or other disorders causing upper motor neuron lesions such as multiple sclerosis. Clinical studies have shown that abobotulinumtoxinA (AboBoNT-A) therapy reduces upper and lower limb spasticity in adults. However, physicians may administer potentially inadequate doses, given the lack of consensus on adjusting dose according to muscle volume, the wide dose ranges in the summary of product characteristics or cited in the published literature, and/or the high quantity of toxin available for injection. Against this background, a systematic literature review based on searches of MEDLINE and Embase (via Ovid SP) and three relevant conferences (2018 to 2020) was conducted in November 2020 to examine AboBoNT-A doses given to adults for upper or lower limb muscles affected by spasticity of any etiology in clinical and real-world evidence studies. From the 1781 unique records identified from the electronic databases and conference proceedings screened, 49 unique studies represented across 56 publications (53 full-text articles, 3 conference abstracts) were eligible for inclusion. Evidence from these studies suggested that AboBoNT-A dose given per muscle in clinical practice varies considerably, with only a slight trend toward a relationship between dose and muscle volume. Expert-based consensus is needed to inform recommendations for standardizing AboBoNT-A treatment initiation doses based on muscle volume.
Keywords: botulinum toxins; central nervous system diseases; injections; intramuscular; muscle hypertonia; muscle spasticity.
Conflict of interest statement
This study was sponsored by Ipsen. AF, II, KF and LL are employees of Evidera, which received funding from Ipsen for performing the SLR and statistical analyses that informed this study. CD, JYL and AFo are employees of Ipsen. DG and AS report personal fees for consultancy from Ipsen, Allergan and Merz.
Figures

References
-
- Javed M., Ali M. Epidemiological Burden of Lower Limb Spasticity in Adults: A Systematic Review. J. Med. Res. Innov. 2019;4:e000195. doi: 10.32892/jmri.195. - DOI
-
- Agence nationale de sécurité du médicament et des produits de santé (ANSM)DYSPORT. [(accessed on 12 December 2021)]. Available online: http://agence-prd.ansm.sante.fr/php/ecodex/frames.php?specid=60242321&ty....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

