Ochratoxin A Defective Aspergillus carbonarius Mutants as Potential Biocontrol Agents
- PMID: 36355995
- PMCID: PMC9695793
- DOI: 10.3390/toxins14110745
Ochratoxin A Defective Aspergillus carbonarius Mutants as Potential Biocontrol Agents
Abstract
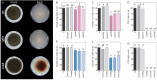
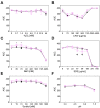
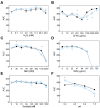
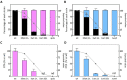
Aspergillus carbonarius is one of the main species responsible for wine, coffee and cocoa toxin contamination. The main mycotoxin produced by this fungus, ochratoxin A (OTA), is a secondary metabolite categorized as a possible carcinogen because of its significant nephrotoxicity and immunosuppressive effects. A polyketide synthase gene (otaA) encodes the first enzyme in the OTA biosynthetic pathway. It is known that the filamentous fungi, growth, development and production of secondary metabolites are interconnected processes governed by global regulatory factors whose encoding genes are generally located outside the gene clusters involved in the biosynthesis of each secondary metabolite, such as the veA gene, which forms part of the VELVET complex. Different fungal strains compete for nutrients and space when they infect their hosts, and safer non-mycotoxigenic strains may be able to outcompete mycotoxigenic strains during colonization. To determine the possible utility of biopesticides based on the competitive exclusion of mycotoxigenic strains by non-toxigenic ones, we used A. carbonarius ΔotaA and ΔveA knockout mutants. Our results showed that during both in vitro growth and infection of grapes, non-mycotoxigenic strains could outcompete the wild-type strain. Additionally, the introduction of the non-mycotoxigenic strain led to a drastic decrease in OTA during both in vitro growth and infection of grapes.
Keywords: VELVET complex; competence; mycotoxin; outcompete; polyketide synthase; secondary metabolism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- EPA . Biopesticide Registration Action Document: Aspergillus flavus AF36. EPA; Washington, DC, USA: 2003. US Environmental Protection Agency Office of Pesticide Programs. PC Code 006456.
-
- EPA . Biopesticide Registration Action Document Aspergillus flavus (NRRL 21882) (PC Code 006500) EPA; Washington, DC, USA: 2004. US Environmental Protection Agency Office of Pesticide Programs.
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

