Characterization of the Aspergillus flavus Population from Highly Aflatoxin-Contaminated Corn in the United States
- PMID: 36356005
- PMCID: PMC9698142
- DOI: 10.3390/toxins14110755
Characterization of the Aspergillus flavus Population from Highly Aflatoxin-Contaminated Corn in the United States
Abstract
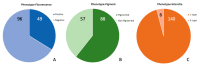
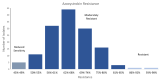
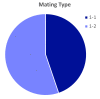
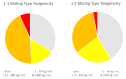
Aflatoxin contamination of corn is a major threat to the safe food and feed. The United States Federal Grain Inspection Service (FGIS) monitors commercial grain shipments for the presence of aflatoxin. A total of 146 Aspergillus flavus were isolated from 29 highly contaminated grain samples to characterize the visual phenotypes, aflatoxin-producing potential, and genotypes to explore the etiological cause of high aflatoxin contamination of US corn. Five of the isolates had reduced sensitivity (43-49% resistant) to the fungicide azoxystrobin, with the remainder all being over 50% resistant to azoxystrobin at the discriminating dose of 2.5 µg/mL. Only six isolates of the highly aflatoxigenic S morphotype were found, and 48 isolates were non-aflatoxigenic. Analysis of the mating type locus revealed 45% MAT 1-1 and 55% MAT 1-2. The A. flavus population originating from the highly aflatoxin contaminated grain samples was compared to a randomly selected subset of isolates originating from commercial corn samples with typical levels of aflatoxin contamination (average < 50 ppb). Use of simple sequence repeat (SSR) genotyping followed by principal component analysis (PCoA) revealed a similar pattern of genotypic distribution in the two populations, but greater diversity in the FGIS-derived population. The noticeable difference between the two populations was that genotypes identical to strain NRRL 21882, the active component of the aflatoxin biocontrol product Afla-Guard™, were ten times more common in the commercial corn population of A. flavus compared to the population from the high-aflatoxin corn samples. The other similarities between the two populations suggest that high aflatoxin concentrations in corn grain are generally the result of infection with common A. flavus genotypes.
Keywords: fungicide resistance; maize; mating type; mycotoxin; population genetics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- IARC . International Agency for Research on Cancer: Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 56. IARC Press; Lyon, France: 1993. [(accessed on 27 October 2022)]. Some naturally occurring substances: Food items and constituents, heterocyclic aromatic amines and mycotoxins.599p. Available online: https://www.ncbi.nlm.nih.gov/books/NBK513574/
-
- Bandyopadhyay R., Ortega-Beltran A., Akande A., Mutegi C., Atehnkeng J., Kaptoge L., Senghor A.L., Adhikari B.N., Cotty P.J. Biological control of aflatoxins in Africa: Current status and potential challenges in the face of climate change. World Mycotoxin J. 2016;9:771–789. doi: 10.3920/WMJ2016.2130. - DOI
-
- Barug D., Bhatnagar D., van Egmond H.P., Van Der Kamp J.W., Van Osenbruggen W.A., Visconti A., editors. The Mycotoxin Factbook: Food & Feed Topics. Wageningen Academic Publishers; Wageningen, The Netherlands: 2006. - DOI
-
- Binder E.M., Krska R. Romer Labs Guide to Mycotoxins. Anytime Publishing Services; Leichestershire, UK: 2012.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

