Long-range axonal projections of transplanted mouse embryonic stem cell-derived hypothalamic neurons into adult mouse brain
- PMID: 36356043
- PMCID: PMC9648832
- DOI: 10.1371/journal.pone.0276694
Long-range axonal projections of transplanted mouse embryonic stem cell-derived hypothalamic neurons into adult mouse brain
Abstract
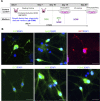
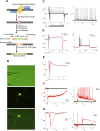
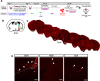
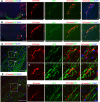
The hypothalamus is comprised of heterogenous cell populations and includes highly complex neural circuits that regulate the autonomic nerve system. Its dysfunction therefore results in severe endocrine disorders. Although recent experiments have been conducted for in vitro organogenesis of hypothalamic neurons from embryonic stem (ES) or induced pluripotent stem (iPS) cells, whether these stem cell-derived hypothalamic neurons can be useful for regenerative medicine remains unclear. We therefore performed orthotopic transplantation of mouse ES cell (mESC)-derived hypothalamic neurons into adult mouse brains. We generated electrophysiologically functional hypothalamic neurons from mESCs and transplanted them into the supraoptic nucleus of mice. Grafts extended their axons along hypothalamic nerve bundles in host brain, and some of them even projected into the posterior pituitary (PPit), which consists of distal axons of the magnocellular neurons located in hypothalamic supraoptic and paraventricular nuclei. The axonal projections to the PPit were not observed when the mESC-derived hypothalamic neurons were ectopically transplanted into the substantia nigra reticular part. These findings suggest that our stem cell-based orthotopic transplantation approach might contribute to the establishment of regenerative medicine for hypothalamic and pituitary disorders.
Copyright: © 2022 Kawata et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Cellular localization and differential distribution of GABAA receptor subunit proteins and messenger RNAs within hypothalamic magnocellular neurons.Neuroscience. 1995 Feb;64(4):1129-43. doi: 10.1016/0306-4522(94)00402-q. Neuroscience. 1995. PMID: 7753380
-
Trigeminohypothalamic and reticulohypothalamic tract neurons in the upper cervical spinal cord and caudal medulla of the rat.J Neurophysiol. 2000 Oct;84(4):2078-112. doi: 10.1152/jn.2000.84.4.2078. J Neurophysiol. 2000. PMID: 11024099
-
Neuropeptide cells and fibers in the hypothalamus and pituitary of the fetal sheep: comparison of oxytocin and arginine vasopressin.Neuroendocrinology. 1989 Dec;50(6):633-43. doi: 10.1159/000125292. Neuroendocrinology. 1989. PMID: 2515463
-
Recapitulating Hypothalamus and Pituitary Development Using Embryonic Stem/Induced Pluripotent Stem Cells.2016 Jul 27. In: Pfaff D, Christen Y, editors. Stem Cells in Neuroendocrinology [Internet]. Cham (CH): Springer; 2016. 2016 Jul 27. In: Pfaff D, Christen Y, editors. Stem Cells in Neuroendocrinology [Internet]. Cham (CH): Springer; 2016. PMID: 28590699 Free Books & Documents. Review.
-
Estrogen modulates oxytocin gene expression in regions of the rat supraoptic and paraventricular nuclei that contain estrogen receptor-beta.Prog Brain Res. 2002;139:15-29. doi: 10.1016/s0079-6123(02)39004-6. Prog Brain Res. 2002. PMID: 12436923 Review.
Cited by
-
Implications of regional identity for neural stem and progenitor cell transplantation in the injured or diseased nervous system.Neural Regen Res. 2024 Apr;19(4):715-716. doi: 10.4103/1673-5374.382236. Neural Regen Res. 2024. PMID: 37843199 Free PMC article. No abstract available.
References
-
- Shapiro M, Weiss JP. Diabetes Insipidus: A Review. J Diabetes Metab. 2012;s6: 2–11. doi: 10.4172/2155-6156.S6-009 - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

