SARS-CoV-2 spike conformation determines plasma neutralizing activity elicited by a wide panel of human vaccines
- PMID: 36356052
- PMCID: PMC9765460
- DOI: 10.1126/sciimmunol.adf1421
SARS-CoV-2 spike conformation determines plasma neutralizing activity elicited by a wide panel of human vaccines
Abstract
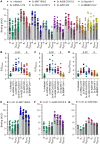
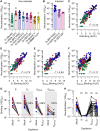
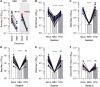
Numerous safe and effective coronavirus disease 2019 vaccines have been developed worldwide that use various delivery technologies and engineering strategies. We show here that vaccines containing prefusion-stabilizing S mutations elicit antibody responses in humans with enhanced recognition of S and the S1 subunit relative to postfusion S as compared with vaccines lacking these mutations or natural infection. Prefusion S and S1 antibody binding titers positively and equivalently correlated with neutralizing activity, and depletion of S1-directed antibodies completely abrogated plasma neutralizing activity. We show that neutralizing activity is almost entirely directed to the S1 subunit and that variant cross-neutralization is mediated solely by receptor binding domain-specific antibodies. Our data provide a quantitative framework for guiding future S engineering efforts to develop vaccines with higher resilience to the emergence of variants than current technologies.
Figures
Update of
-
SARS-CoV-2 spike conformation determines plasma neutralizing activity.bioRxiv [Preprint]. 2021 Dec 21:2021.12.19.473391. doi: 10.1101/2021.12.19.473391. bioRxiv. 2021. Update in: Sci Immunol. 2022 Dec 23;7(78):eadf1421. doi: 10.1126/sciimmunol.adf1421. PMID: 34981060 Free PMC article. Updated. Preprint.
References
-
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T. S., Herrler G., Wu N. H., Nitsche A., Müller M. A., Drosten C., Pöhlmann S., SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181, 271–280.e8 (2020). - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

