TPP1 promoter mutations cooperate with TERT promoter mutations to lengthen telomeres in melanoma
- PMID: 36356143
- PMCID: PMC10590476
- DOI: 10.1126/science.abq0607
TPP1 promoter mutations cooperate with TERT promoter mutations to lengthen telomeres in melanoma
Abstract
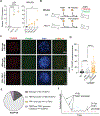
Overcoming replicative senescence is an essential step during oncogenesis, and the reactivation of TERT through promoter mutations is a common mechanism. TERT promoter mutations are acquired in about 75% of melanomas but are not sufficient to maintain telomeres, suggesting that additional mutations are required. We identified a cluster of variants in the promoter of ACD encoding the shelterin component TPP1. ACD promoter variants are present in about 5% of cutaneous melanoma and co-occur with TERT promoter mutations. The two most common somatic variants create or modify binding sites for E-twenty-six (ETS) transcription factors, similar to mutations in the TERT promoter. The variants increase the expression of TPP1 and function together with TERT to synergistically lengthen telomeres. Our findings suggest that TPP1 promoter variants collaborate with TERT activation to enhance telomere maintenance and immortalization in melanoma.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
JMK reports advisory/consultancy roles with Amgen Inc., Ankyra Therapeutics, Applied Clinical Intelligence LLC, Axio Research LLC, Becker Pharmaceutical Consulting, Bristol Myers Squibb, Cancer Network, Checkmate Pharmaceuticals, DermTech, Fenix Group International, Harbour BioMed, Immunocore LLC, iOnctura, Istari Oncology, Magnolia Innovations LLC, Merck, Natera Inc, Novartis Pharmaceuticals, OncoCyte Corporation, OncoSec Medical Inc., PathAI Inc., Pfizer Inc., Replimune, Scopus BioPharma, SR One Capital Management, Takeda Development Center Americas Inc., and Takeda Pharmaceutical Company Limited; and research grant/funding to their institution from Amgen Inc., Bristol Myers Squibb, Checkmate Pharmaceuticals, Harbour BioMed, Immvira Pharma Co., Immunocore LLC, Iovance Biotherapeutics, Novartis Pharmaceuticals, Takeda, and Verastem Inc.
All other authors have no conflicts of interest to declare.
Figures

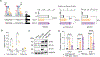
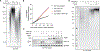
References
-
- Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW, Specific association of human telomerase activity with immortal cells and cancer. Science. 266, 2011–2015 (1994). - PubMed
-
- Hanahan D, Weinberg RA, Hallmarks of cancer: the next generation. Cell. 144, 646–674 (2011). - PubMed
-
- Harley CB, Futcher AB, Greider CW, Telomeres shorten during ageing of human fibroblasts. Nature. 345, 458–460 (1990). - PubMed
-
- Wu KJ, Grandori C, Amacker M, Simon-Vermot N, Polack A, Lingner J, DallaFavera R, Direct activation of TERT transcription by c-MYC. Nat. Genet 21, 220–224 (1999). - PubMed
-
- Horn S, Figl A, Rachakonda PS, Fischer C, Sucker A, Gast A, Kadel S, Moll I, Nagore E, Hemminki K, Schadendorf D, Kumar R, TERT promoter mutations in familial and sporadic melanoma. Science. 339, 959–961 (2013). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

