Secreted mutant calreticulins as rogue cytokines in myeloproliferative neoplasms
- PMID: 36356299
- PMCID: PMC10651872
- DOI: 10.1182/blood.2022016846
Secreted mutant calreticulins as rogue cytokines in myeloproliferative neoplasms
Abstract
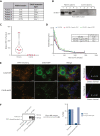
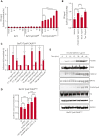
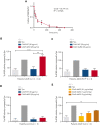
Mutant calreticulin (CALR) proteins resulting from a -1/+2 frameshifting mutation of the CALR exon 9 carry a novel C-terminal amino acid sequence and drive the development of myeloproliferative neoplasms (MPNs). Mutant CALRs were shown to interact with and activate the thrombopoietin receptor (TpoR/MPL) in the same cell. We report that mutant CALR proteins are secreted and can be found in patient plasma at levels up to 160 ng/mL, with a mean of 25.64 ng/mL. Plasma mutant CALR is found in complex with soluble transferrin receptor 1 (sTFR1) that acts as a carrier protein and increases mutant CALR half-life. Recombinant mutant CALR proteins bound and activated the TpoR in cell lines and primary megakaryocytic progenitors from patients with mutated CALR in which they drive thrombopoietin-independent colony formation. Importantly, the CALR-sTFR1 complex remains functional for TpoR activation. By bioluminescence resonance energy transfer assay, we show that mutant CALR proteins produced in 1 cell can specifically interact in trans with the TpoR on a target cell. In comparison with cells that only carry TpoR, cells that carry both TpoR and mutant CALR are hypersensitive to exogenous mutant CALR proteins and respond to levels of mutant CALR proteins similar to those in patient plasma. This is consistent with CALR-mutated cells that expose TpoR carrying immature N-linked sugars at the cell surface. Thus, secreted mutant CALR proteins will act more specifically on the MPN clone. In conclusion, a chaperone, CALR, can turn into a rogue cytokine through somatic mutation of its encoding gene.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: R.K. and S.N.C. and are cofounders of MyeloPro GmbH. The remaining authors declare no competing financial interests.
Figures





Comment in
-
CALR goes rogue.Blood. 2023 Feb 23;141(8):818-820. doi: 10.1182/blood.2022018788. Blood. 2023. PMID: 36821187 No abstract available.
References
-
- Klampfl T, Gisslinger H, Harutyunyan AS, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med. 2013;369(25):2379–2390. - PubMed
-
- Michalak M, Groenendyk J, Szabo E, Gold LI, Opas M. Calreticulin, a multi-process calcium-buffering chaperone of the endoplasmic reticulum. Biochem J. 2009;417(3):651–666. - PubMed
-
- Chachoua I, Pecquet C, El-Khoury M, et al. Thrombopoietin receptor activation by myeloproliferative neoplasm associated calreticulin mutants. Blood. 2016;127(10):1325–1335. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

