BI 456906: Discovery and preclinical pharmacology of a novel GCGR/GLP-1R dual agonist with robust anti-obesity efficacy
- PMID: 36356832
- PMCID: PMC9679702
- DOI: 10.1016/j.molmet.2022.101633
BI 456906: Discovery and preclinical pharmacology of a novel GCGR/GLP-1R dual agonist with robust anti-obesity efficacy
Abstract
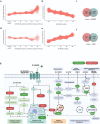
Objective: Obesity and its associated comorbidities represent a global health challenge with a need for well-tolerated, effective, and mechanistically diverse pharmaceutical interventions. Oxyntomodulin is a gut peptide that activates the glucagon receptor (GCGR) and glucagon-like peptide-1 receptor (GLP-1R) and reduces bodyweight by increasing energy expenditure and reducing energy intake in humans. Here we describe the pharmacological profile of the novel glucagon receptor (GCGR)/GLP-1 receptor (GLP-1R) dual agonist BI 456906.
Methods: BI 456906 was characterized using cell-based in vitro assays to determine functional agonism. In vivo pharmacological studies were performed using acute and subchronic dosing regimens to demonstrate target engagement for the GCGR and GLP-1R, and weight lowering efficacy.
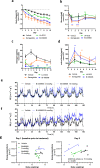
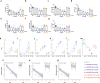
Results: BI 456906 is a potent, acylated peptide containing a C18 fatty acid as a half-life extending principle to support once-weekly dosing in humans. Pharmacological doses of BI 456906 provided greater bodyweight reductions in mice compared with maximally effective doses of the GLP-1R agonist semaglutide. BI 456906's superior efficacy is the consequence of increased energy expenditure and reduced food intake. Engagement of both receptors in vivo was demonstrated via glucose tolerance, food intake, and gastric emptying tests for the GLP-1R, and liver nicotinamide N-methyltransferase mRNA expression and circulating biomarkers (amino acids, fibroblast growth factor-21) for the GCGR. The dual activity of BI 456906 at the GLP-1R and GCGR was supported using GLP-1R knockout and transgenic reporter mice, and an ex vivo bioactivity assay.
Conclusions: BI 456906 is a potent GCGR/GLP-1R dual agonist with robust anti-obesity efficacy achieved by increasing energy expenditure and decreasing food intake.
Keywords: G protein coupled receptor; Glucagon; Glucagon-like peptide-1; Obesity; Peptide.
Copyright © 2022 Boehringer Ingelheim Pharma GmbH & CoKG. Published by Elsevier GmbH.. All rights reserved.
Figures





References
-
- Hales C.M., Carroll M.D., Fryar C.D., Ogden C.L. Prevalence of obesity and severe obesity among adults: United States, 2017-2018. NCHS Data Brief. 2020;(360):1–8. - PubMed
-
- World Health Organisation . 2017. Prevalence of obesity among adults, BMI ≥ 30, age-standardised estimates by WHO region.http://apps.who.int/gho/data/view.main.REGION2480A Available at:
-
- Rinella M.E. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313(22):2263–2273. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

