Functional consequences of short-term exposure to opioids versus cannabinoids in nonhuman primates
- PMID: 36356937
- PMCID: PMC9742330
- DOI: 10.1016/j.neuropharm.2022.109328
Functional consequences of short-term exposure to opioids versus cannabinoids in nonhuman primates
Abstract
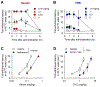
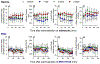
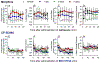
Opioids provide pain relief but are associated with several adverse effects. Researchers are exploring cannabis-based medicine as an alternative. However, little is known about the tendency for physical dependence on cannabinoids in comparison with that on opioids in primates. The aim of this study was to compare the potency of heroin and delta-9-tetrahydrocannabinol (THC) in eliciting analgesic effects and the development of physical dependence between opioids and cannabinoids in both male and female rhesus monkeys. Systemic administration of either heroin (0.03-0.18 mg/kg) or THC (0.3-1.8 mg/kg) in a dose-dependent manner produced antinociceptive effects against an acute thermal nociceptive stimulus. The μ-opioid receptor antagonist naltrexone (0.01 mg/kg) and the cannabinoid receptor antagonist SR141716A (0.3 mg/kg) produced the same degree of rightward shift in the dose-response curves for heroin- and THC-induced antinociception, respectively. Monkeys implanted with telemetry devices were subjected to short-term repeated administrations (two injections per day for 1-3 days) of either heroin (0.18 mg/kg), morphine (1.8 mg/kg), THC (1.8 mg/kg), or CP 55,940 (0.032 mg/kg). Administration of naltrexone (0.01 mg/kg) increased respiration, heart rate, and blood pressure in heroin- or morphine-treated monkeys. In contrast, administration of SR141716A (0.3 mg/kg) did not cause a significant change in these physiological parameters in THC- or CP 55,940-treated monkeys. Additionally, morphine, but not CP 55,940, enhanced the monkeys' hypersensitivity to the algogen capsaicin. Collectively, these results demonstrate that in nonhuman primates, both opioids and cannabinoids exert comparable antinociception; however, physical dependence on opioids, but not cannabinoids, at their antinociceptive doses, occurs following short-term exposures.
Keywords: Antinociception; CP 55,940; Heroin; Hypersensitivity; Morphine; Physical dependence; THC.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of competing interest None.
Figures
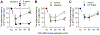
References
-
- Abuse NIoD (2019). Opioid Overdose Crisis. Opioid Overdose Crisis.
-
- Angst MS, & Clark JD (2006). Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology 104: 570–587. - PubMed
-
- Aviram J, Pud D, Gershoni T, Schiff-Keren B, Ogintz M, Vulfsons S, et al. (2021). Medical cannabis treatment for chronic pain: Outcomes and prediction of response. Eur J Pain 25: 359–374. - PubMed
-
- Aviram J, & Samuelly-Leichtag G (2017). Efficacy of Cannabis-Based Medicines for Pain Management: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Pain Physician 20: E755–E796. - PubMed
-
- Azorlosa JL (1994). The effect of chronic naltrexone pretreatment on associative vs. non-associative morphine tolerance. Drug Alcohol Depend 36: 65–67. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

