Studying the Long-term Impact of COVID-19 in Kids (SLICK). Healthcare use and costs in children and young people following community-acquired SARS-CoV-2 infection: protocol for an observational study using linked primary and secondary routinely collected healthcare data from England, Scotland and Wales
- PMID: 36356998
- PMCID: PMC9659708
- DOI: 10.1136/bmjopen-2022-063271
Studying the Long-term Impact of COVID-19 in Kids (SLICK). Healthcare use and costs in children and young people following community-acquired SARS-CoV-2 infection: protocol for an observational study using linked primary and secondary routinely collected healthcare data from England, Scotland and Wales
Abstract
Introduction: SARS-CoV-2 infection rarely causes hospitalisation in children and young people (CYP), but mild or asymptomatic infections are common. Persistent symptoms following infection have been reported in CYP but subsequent healthcare use is unclear. We aim to describe healthcare use in CYP following community-acquired SARS-CoV-2 infection and identify those at risk of ongoing healthcare needs.
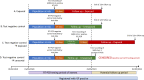
Methods and analysis: We will use anonymised individual-level, population-scale national data linking demographics, comorbidities, primary and secondary care use and mortality between 1 January 2019 and 1 May 2022. SARS-CoV-2 test data will be linked from 1 January 2020 to 1 May 2022. Analyses will use Trusted Research Environments: OpenSAFELY in England, Secure Anonymised Information Linkage (SAIL) Databank in Wales and Early Pandemic Evaluation and Enhanced Surveillance of COVID-19 in Scotland (EAVE-II). CYP aged ≥4 and <18 years who underwent SARS-CoV-2 reverse transcription PCR (RT-PCR) testing between 1 January 2020 and 1 May 2021 and those untested CYP will be examined.The primary outcome measure is cumulative healthcare cost over 12 months following SARS-CoV-2 testing, stratified into primary or secondary care, and physical or mental healthcare. We will estimate the burden of healthcare use attributable to SARS-CoV-2 infections in the 12 months after testing using a matched cohort study of RT-PCR positive, negative or untested CYP matched on testing date, with adjustment for confounders. We will identify factors associated with higher healthcare needs in the 12 months following SARS-CoV-2 infection using an unmatched cohort of RT-PCR positive CYP. Multivariable logistic regression and machine learning approaches will identify risk factors for high healthcare use and characterise patterns of healthcare use post infection.
Ethics and dissemination: This study was approved by the South-Central Oxford C Health Research Authority Ethics Committee (13/SC/0149). Findings will be preprinted and published in peer-reviewed journals. Analysis code and code lists will be available through public GitHub repositories and OpenCodelists with meta-data via HDR-UK Innovation Gateway.
Keywords: COVID-19; epidemiology; health economics; paediatric infectious disease & immunisation.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: OVS reports an institutional payment from HDR-UK/Alan Turing for work on this study. LAT reports institutional contracts with UKRI, NIHR, MRC, institutional consulting fees from Bayer, support to attend MHRA meetings and unpaid membership of two non-industry funded trial advisory committees. MJS reports an institutional payment from HDR-UK/Alan Turing for work on this study. CRS reports institutional grants from MBIE, HRC and MRC. SVK reports funding from NRS, MRC and the Scottish Government Chief Scientist Office. He was co-chair of the Scottish Government’s Expert Reference Group on Ethnicity and COVID-19 and a member of the UK Scientific Advisory Group on Emergencies subgroup on ethnicity. MGS reports grants from NIHR, MRC and Health Protection Research Unit in Emerging & Zoonotic Infections, University of Liverpool. He also reports a role as Independent external and non-remunerated member of Pfizer’s External Data Monitoring Committee for their mRNA vaccine program. He is Chair of Infectious Disease Scientific Advisory Board for Integrum Scientific LLC, Greensboro, NC, USA and director of MedEx Solutions Ltd. He reports minority stock ownership for Integrum Scientific LLC, Greensboro, NC, USA and majority stock ownership for MedEx Solutions Ltd. He also reports a gift from Chiesi Farmaceutici SPA to his institution of a clinical trial investigational medicinal product without encumbrance and distribution of same to trial sites. He is also a non-remunerated independent member of HMG UK Scientific Advisory Group for Emergencies (SAGE, COVID-19 Response) and HMG UK New Emerging Respiratory Virus Threats Advisory Group (NERVTAG). SB has received an institutional payment from HDR-UK/Alan Turing funding UOE Ref: 11563729 for work on this study. She also reports institutional payments from MRC, Welsh Government and NIHR. She is a member of the Population and Systems Medicine MRC board. AS reports an institutional payment from HDR-UK/Alan Turing and research grants for EAVE II and BREATHE Hub. He also reports non-remunerated positions on AstraZeneca’s Thrombotic Thrombocytopenic Taskforce and Scottish and UK Government Advisory Committees. RAL is a member of the Welsh Government COVID-19 Technical Advisory Group. BG has received research funding from HDRUK, the Laura and John Arnold Foundation, the Wellcome Trust, the NIHR Oxford Biomedical Research Centre, the NHS National Institute for Health Research School of Primary Care Research, the Mohn-Westlake Foundation, the Good Thinking Foundation, the Health Foundation, and the World Health Organisation; he also receives personal income from speaking and writing for lay audiences on the misuse of science.
Figures
Similar articles
-
Uptake, effectiveness and safety of COVID-19 vaccines in children and young people in Scotland: Protocol for early pandemic evaluation and enhanced surveillance of COVID-19 (EAVE II).J Glob Health. 2021 Dec 25;11:05026. doi: 10.7189/jogh.11.05026. eCollection 2021. J Glob Health. 2021. PMID: 35003715 Free PMC article.
-
Investigating the uptake, effectiveness and safety of COVID-19 vaccines: protocol for an observational study using linked UK national data.BMJ Open. 2022 Feb 14;12(2):e050062. doi: 10.1136/bmjopen-2021-050062. BMJ Open. 2022. PMID: 35165107 Free PMC article.
-
Early Pandemic Evaluation and Enhanced Surveillance of COVID-19 (EAVE II): protocol for an observational study using linked Scottish national data.BMJ Open. 2020 Jun 21;10(6):e039097. doi: 10.1136/bmjopen-2020-039097. BMJ Open. 2020. PMID: 32565483 Free PMC article.
-
Antibody tests for identification of current and past infection with SARS-CoV-2.Cochrane Database Syst Rev. 2020 Jun 25;6(6):CD013652. doi: 10.1002/14651858.CD013652. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2022 Nov 17;11:CD013652. doi: 10.1002/14651858.CD013652.pub2. PMID: 32584464 Free PMC article. Updated.
-
Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2021 Mar 24;3(3):CD013705. doi: 10.1002/14651858.CD013705.pub2. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 Jul 22;7:CD013705. doi: 10.1002/14651858.CD013705.pub3. PMID: 33760236 Free PMC article. Updated.
References
-
- Birrel P, Blake J, van Leeuwen E. Report on Nowcasting and Forecasting - 9th December 2021 MRC Biostatistics Unit; 2022. https://www.mrc-bsu.cam.ac.uk/now-casting/report-on-nowcasting-and-forec... [Accessed 9 Feb 2022].
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
