Sugarcane cultivation practices modulate rhizosphere microbial community composition and structure
- PMID: 36357461
- PMCID: PMC9649670
- DOI: 10.1038/s41598-022-23562-6
Sugarcane cultivation practices modulate rhizosphere microbial community composition and structure
Abstract
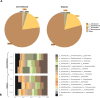
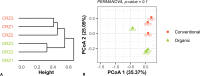
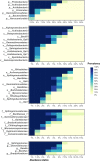
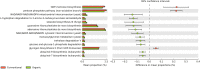
Sugarcane (Saccharum spp.) represents a crop of great economic importance, remarkably relevant in the food industry and energy supply chains from renewable sources. However, its conventional cultivation involves the intensive use of fertilizers, pesticides, and other agrochemical agents whose detrimental effects on the environment are notorious. Alternative systems, such as organic farming, have been presented as an environmentally friendly way of production. Still, the outcomes of different cropping systems on the microbiota associated with sugarcane-whose role in its health and growth is crucial-remain underexplored. Thus, we studied the rhizospheric microbiota of two adjacent sugarcane fields, which differ in terms of the type of farming system. For this, we used the sequencing of taxonomic markers of prokaryotes (gene 16S rRNA, subregions V3-V4) and fungi (Internal transcribed spacer 2) and evaluated the changes caused by the systems. Our results show a well-conserved microbiota composition among farming systems in the highest taxonomic ranks, such as phylum, class, and order. Also, both systems showed very similar alpha diversity indices and shared core taxa with growth-promoting capacities, such as bacteria from the Bacillus and Bradyrhizobium genera and the fungal genus Trichoderma. However, the composition at more specific levels denotes differences, such as the separation of the samples concerning beta diversity and the identification of 74 differentially abundant taxa between the systems. Of these, 60 were fungal taxa, indicating that this microbiota quota is more susceptible to changes caused by farming systems. The analysis of co-occurrence networks also showed the formation of peripheral sub-networks associated with the treatments-especially in fungi-and the presence of keystone taxa in terms of their ability to mediate relationships between other members of microbial communities. Considering that both crop fields used the same cultivar and had almost identical soil properties, we conclude that the observed findings are effects of the activities intrinsic to each system and can contribute to a better understanding of the effects of farming practices on the plant microbiome.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures








Similar articles
-
Farming systems influence the compositional, structural, and functional characteristics of the sugarcane-associated microbiome.Microbiol Res. 2021 Nov;252:126866. doi: 10.1016/j.micres.2021.126866. Epub 2021 Sep 9. Microbiol Res. 2021. PMID: 34536678
-
Rhizosphere Bacterial Community Characteristics over Different Years of Sugarcane Ratooning in Consecutive Monoculture.Biomed Res Int. 2019 Nov 11;2019:4943150. doi: 10.1155/2019/4943150. eCollection 2019. Biomed Res Int. 2019. PMID: 31815142 Free PMC article.
-
Inter-Kingdom Networks of Canola Microbiome Reveal Bradyrhizobium as Keystone Species and Underline the Importance of Bulk Soil in Microbial Studies to Enhance Canola Production.Microb Ecol. 2022 Nov;84(4):1166-1181. doi: 10.1007/s00248-021-01905-6. Epub 2021 Nov 2. Microb Ecol. 2022. PMID: 34727198
-
Structure and variation of root-associated microbiomes of potato grown in alfisol.World J Microbiol Biotechnol. 2019 Nov 14;35(12):181. doi: 10.1007/s11274-019-2761-3. World J Microbiol Biotechnol. 2019. PMID: 31728652
-
Rhizospheric microbiome: Bio-based emerging strategies for sustainable agriculture development and future perspectives.Microbiol Res. 2022 Jan;254:126901. doi: 10.1016/j.micres.2021.126901. Epub 2021 Oct 23. Microbiol Res. 2022. PMID: 34700186 Review.
Cited by
-
Reproducing plant microbiome research reveals site and time as key drivers of apple tree phyllosphere bacterial communities.Sci Rep. 2025 Jul 15;15(1):25620. doi: 10.1038/s41598-025-10729-0. Sci Rep. 2025. PMID: 40664985 Free PMC article.
-
Bioprospecting Microbial Consortia for Tebuthiuron Degradation in Agricultural Soils: An Alternative Bayesian-Driven Colorimetric Protocol.Environ Microbiol Rep. 2025 Jun;17(3):e70109. doi: 10.1111/1758-2229.70109. Environ Microbiol Rep. 2025. PMID: 40418115 Free PMC article.
References
-
- Meghana M, Shastri Y. Sustainable valorization of sugar industry waste: Status, opportunities, and challenges. Biores. Technol. 2020;303:122929. - PubMed
-
- Kassam A, Friedrich T, Shaxson F, Pretty J. The spread of conservation agriculture: justification, sustainability and uptake. Int. J. Agric. Sustain. 2009;7:292–320.
-
- Malviya MK, Solanki M K, Li C-N, Htun R, Singh RK, Singh P, Li Y-R. Microbiomes and Plant Health. Elsevier; 2021. Sugarcane microbiome: role in sustainable production; pp. 225–242.
-
- Sandhu HS, Wratten SD, Cullen R. Organic agriculture and ecosystem services. Environ. Sci. Policy. 2010;13:1–7.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

