Ceragenin CSA-13 displays high antibacterial efficiency in a mouse model of urinary tract infection
- PMID: 36357517
- PMCID: PMC9649698
- DOI: 10.1038/s41598-022-23281-y
Ceragenin CSA-13 displays high antibacterial efficiency in a mouse model of urinary tract infection
Abstract
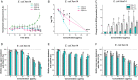
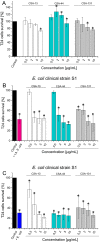
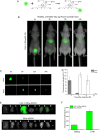
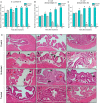
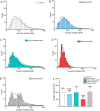
Ceragenins (CSAs) are synthetic, lipid-based molecules that display activities of natural antimicrobial peptides. Previous studies demonstrated their high in vitro activity against pathogens causing urinary tract infections (UTIs), but their efficiency in vivo was not explored to date. In this study, we aimed to investigate the bactericidal efficiency of ceragenins against E. coli (Xen14 and clinical UPEC strains) isolates both in vitro and in vivo, as well to explore CSA-13 biodistribution and ability to modulate nanomechanical alterations of infected tissues using animal model of UTI. CSA-44, CSA-131 and particularly CSA-13 displayed potent bactericidal effect against tested E. coli strains, and this effect was mediated by induction of oxidative stress. Biodistribution studies indicated that CSA-13 accumulates in kidneys and liver and is eliminated with urine and bile acid. We also observed that ceragenin CSA-13 reverses infection-induced alterations in mechanical properties of mouse bladders tissue, which confirms the preventive role of CSA-13 against bacteria-induced tissue damage and potentially promote the restoration of microenvironment with biophysical features unfavorable for bacterial growth and spreading. These data justify the further work on employment of CSA-13 in the treatment of urinary tract infections.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

