Polymerization of the backbone of the pectic polysaccharide rhamnogalacturonan I
- PMID: 36357524
- PMCID: PMC10115348
- DOI: 10.1038/s41477-022-01270-3
Polymerization of the backbone of the pectic polysaccharide rhamnogalacturonan I
Erratum in
-
Author Correction: Polymerization of the backbone of the pectic polysaccharide rhamnogalacturonan I.Nat Plants. 2023 Apr;9(4):673. doi: 10.1038/s41477-023-01395-z. Nat Plants. 2023. PMID: 36941371 Free PMC article. No abstract available.
Abstract
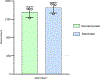
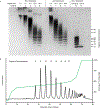
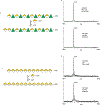
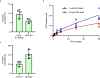
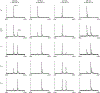
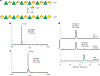
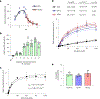
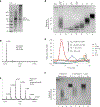
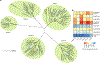
Rhamnogalacturonan I (RG-I) is a major plant cell wall pectic polysaccharide defined by its repeating disaccharide backbone structure of [4)-α-D-GalA-(1,2)-α-L-Rha-(1,]. A family of RG-I:Rhamnosyltransferases (RRT) has previously been identified, but synthesis of the RG-I backbone has not been demonstrated in vitro because the identity of Rhamnogalacturonan I:Galaturonosyltransferase (RG-I:GalAT) was unknown. Here a putative glycosyltransferase, At1g28240/MUCI70, is shown to be an RG-I:GalAT. The name RGGAT1 is proposed to reflect the catalytic activity of this enzyme. When incubated together with the rhamnosyltransferase RRT4, the combined activities of RGGAT1 and RRT4 result in elongation of RG-I acceptors in vitro into a polymeric product. RGGAT1 is a member of a new GT family categorized as GT116, which does not group into existing GT-A clades and is phylogenetically distinct from the GALACTURONOSYLTRANSFERASE (GAUT) family of GalA transferases that synthesize the backbone of the pectin homogalacturonan. RGGAT1 has a predicted GT-A fold structure but employs a metal-independent catalytic mechanism that is rare among glycosyltransferases with this fold type. The identification of RGGAT1 and the 8-member Arabidopsis GT116 family provides a new avenue for studying the mechanism of RG-I synthesis and the function of RG-I in plants.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures
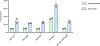




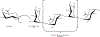

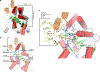
Similar articles
-
Pectin RG-I rhamnosyltransferases represent a novel plant-specific glycosyltransferase family.Nat Plants. 2018 Sep;4(9):669-676. doi: 10.1038/s41477-018-0217-7. Epub 2018 Aug 6. Nat Plants. 2018. PMID: 30082766
-
Biochemical characterization of rhamnosyltransferase involved in biosynthesis of pectic rhamnogalacturonan I in plant cell wall.Biochem Biophys Res Commun. 2017 Apr 22;486(1):130-136. doi: 10.1016/j.bbrc.2017.03.012. Epub 2017 Mar 8. Biochem Biophys Res Commun. 2017. PMID: 28283389
-
Multiple Arabidopsis galacturonosyltransferases synthesize polymeric homogalacturonan by oligosaccharide acceptor-dependent or de novo synthesis.Plant J. 2022 Mar;109(6):1441-1456. doi: 10.1111/tpj.15640. Epub 2021 Dec 27. Plant J. 2022. PMID: 34908202 Free PMC article.
-
The structure, function, and biosynthesis of plant cell wall pectic polysaccharides.Carbohydr Res. 2009 Sep 28;344(14):1879-900. doi: 10.1016/j.carres.2009.05.021. Epub 2009 Jun 2. Carbohydr Res. 2009. PMID: 19616198 Review.
-
Rethinking the impact of RG-I mainly from fruits and vegetables on dietary health.Crit Rev Food Sci Nutr. 2020;60(17):2938-2960. doi: 10.1080/10408398.2019.1672037. Epub 2019 Oct 14. Crit Rev Food Sci Nutr. 2020. PMID: 31607142 Review.
Cited by
-
Analysis of carbohydrates and glycoconjugates by matrix-assisted laser desorption/ionization mass spectrometry: An update for 2021-2022.Mass Spectrom Rev. 2025 May-Jun;44(3):213-453. doi: 10.1002/mas.21873. Epub 2024 Jun 24. Mass Spectrom Rev. 2025. PMID: 38925550 Free PMC article. Review.
-
A Fresh Look at Celery Collenchyma and Parenchyma Cell Walls Through a Combination of Biochemical, Histochemical, and Transcriptomic Analyses.Int J Mol Sci. 2025 Jan 16;26(2):738. doi: 10.3390/ijms26020738. Int J Mol Sci. 2025. PMID: 39859452 Free PMC article.
-
Putative rhamnogalacturonan-II glycosyltransferase identified through callus gene editing which bypasses embryo lethality.Plant Physiol. 2024 Jul 31;195(4):2551-2565. doi: 10.1093/plphys/kiae259. Plant Physiol. 2024. PMID: 38739546 Free PMC article.
-
Biochemical data documenting variations in mucilage polysaccharides in a range of glycosyltransferase mutants.Sci Data. 2023 Oct 14;10(1):702. doi: 10.1038/s41597-023-02604-2. Sci Data. 2023. PMID: 37838800 Free PMC article.
-
How it all begins: molecular players of the early graviresponse in the non-elongating part of flax stem.Plant Mol Biol. 2025 Apr 26;115(3):61. doi: 10.1007/s11103-025-01588-4. Plant Mol Biol. 2025. PMID: 40285981
References
-
- Atmodjo MA, Hao Z & Mohnen D Evolving views of pectin biosynthesis. Annu. Rev. Plant Biol 64, 747–779 (2013). - PubMed
-
- Biswal AK et al. Sugar release and growth of biofuel crops are improved by downregulation of pectin biosynthesis. Nat. Biotechnol 36, 249–257 (2018). - PubMed
-
- Wu D et al. Dietary pectic substances enhance gut health by its polycomponent: a review. Compr. Rev. Food Sci. Food Saf 20, 2015–2039 (2021). - PubMed
-
- Bonnin E, Garnier C & Ralet MC Pectin-modifying enzymes and pectin-derived materials: applications and impacts. Appl. Microbiol. Biotechnol 98, 519–532 (2014). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

