Chemical inhibition of DPP9 sensitizes the CARD8 inflammasome in HIV-1-infected cells
- PMID: 36357533
- PMCID: PMC10065922
- DOI: 10.1038/s41589-022-01182-5
Chemical inhibition of DPP9 sensitizes the CARD8 inflammasome in HIV-1-infected cells
Abstract
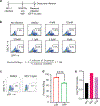
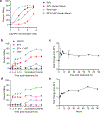
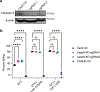
Non-nucleoside reverse transcriptase inhibitors (NNRTIs) induce pyroptosis of HIV-1-infected CD4+ T cells through induction of intracellular HIV-1 protease activity, which activates the CARD8 inflammasome. Because high concentrations of NNRTIs are required for efficient elimination of HIV-1-infected cells, it is important to elucidate ways to sensitize the CARD8 inflammasome to NNRTI-induced activation. We show that this sensitization can be achieved through chemical inhibition of the CARD8 negative regulator DPP9. The DPP9 inhibitor Val-boroPro (VbP) can kill HIV-1-infected cells without the presence of NNRTIs and act synergistically with NNRTIs to promote clearance of HIV-1-infected cells in vitro and in humanized mice. More importantly, VbP is able to enhance clearance of residual HIV-1 in CD4+ T cells isolated from people living with HIV (PLWH). We also show that VbP can partially overcome NNRTI resistance. This offers a promising strategy for enhancing NNRTI efficacy in the elimination of HIV-1 reservoirs in PLWH.
© 2022. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures







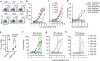




References
-
- Hütter G, et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med 360, 692–698 (2009). - PubMed
-
- Finzi D, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 278, 1295–1300 (1997). - PubMed
-
- Ganor Y, et al. HIV-1 reservoirs in urethral macrophages of patients under suppressive antiretroviral therapy. Nat Microbiol 4, 633–644 (2019). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

