Immune and spermatogenesis-related loci are involved in the development of extreme patterns of male infertility
- PMID: 36357561
- PMCID: PMC9649734
- DOI: 10.1038/s42003-022-04192-0
Immune and spermatogenesis-related loci are involved in the development of extreme patterns of male infertility
Abstract
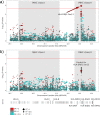
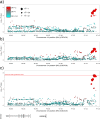
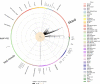
We conducted a genome-wide association study in a large population of infertile men due to unexplained spermatogenic failure (SPGF). More than seven million genetic variants were analysed in 1,274 SPGF cases and 1,951 unaffected controls from two independent European cohorts. Two genomic regions were associated with the most severe histological pattern of SPGF, defined by Sertoli cell-only (SCO) phenotype, namely the MHC class II gene HLA-DRB1 (rs1136759, P = 1.32E-08, OR = 1.80) and an upstream locus of VRK1 (rs115054029, P = 4.24E-08, OR = 3.14), which encodes a protein kinase involved in the regulation of spermatogenesis. The SCO-associated rs1136759 allele (G) determines a serine in the position 13 of the HLA-DRβ1 molecule located in the antigen-binding pocket. Overall, our data support the notion of unexplained SPGF as a complex trait influenced by common variation in the genome, with the SCO phenotype likely representing an immune-mediated condition.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Tournaye H, Krausz C, Oates RD. Novel concepts in the aetiology of male reproductive impairment. Lancet Diabetes Endocrinol. 2017;5:544–553. - PubMed
-
- Krausz C, Riera-Escamilla A. Genetics of male infertility. Nat. Rev. Urol. 2018;15:369–384. - PubMed
-
- Palermo G, et al. Sperm characteristics and outcome of human assisted fertilization by subzonal insemination and intracytoplasmic sperm injection. Fertil. Steril. 1993;59:826–835. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

