An engineered T7 RNA polymerase that produces mRNA free of immunostimulatory byproducts
- PMID: 36357718
- PMCID: PMC10110463
- DOI: 10.1038/s41587-022-01525-6
An engineered T7 RNA polymerase that produces mRNA free of immunostimulatory byproducts
Abstract
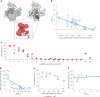
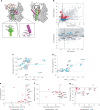
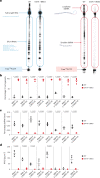
In vitro transcription (IVT) is a DNA-templated process for synthesizing long RNA transcripts, including messenger RNA (mRNA). For many research and commercial applications, IVT of mRNA is typically performed using bacteriophage T7 RNA polymerase (T7 RNAP) owing to its ability to produce full-length RNA transcripts with high fidelity; however, T7 RNAP can also produce immunostimulatory byproducts such as double-stranded RNA that can affect protein expression. Such byproducts require complex purification processes, using methods such as reversed-phase high-performance liquid chromatography, to yield safe and effective mRNA-based medicines. To minimize the need for downstream purification processes, we rationally and computationally engineered a double mutant of T7 RNAP that produces substantially less immunostimulatory RNA during IVT compared with wild-type T7 RNAP. The resulting mutant allows for a simplified production process with similar mRNA potency, lower immunostimulatory content and quicker manufacturing time compared with wild-type T7 RNAP. Herein, we describe the computational design and development of this improved T7 RNAP variant.
© 2022. The Author(s).
Conflict of interest statement
K.R., M.J.M. and A.E.R. are employees of Moderna Inc. A.D. and E.M.H. were employees of Moderna at the time the research was conducted. A.D., K.R., E.M.H., M.J.M. and A.E.R. are shareholders in Moderna. The information discussed in this manuscript is protected by a patent (US Patent 11,066,686) in the United States and in foreign jurisdictions for Moderna, Inc.
Figures


Comment in
-
Improving mRNA production.Nat Rev Drug Discov. 2023 Jan;22(1):19. doi: 10.1038/d41573-022-00200-4. Nat Rev Drug Discov. 2023. PMID: 36450858 No abstract available.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

