Evolution of an adenine base editor into a small, efficient cytosine base editor with low off-target activity
- PMID: 36357719
- PMCID: PMC10188366
- DOI: 10.1038/s41587-022-01533-6
Evolution of an adenine base editor into a small, efficient cytosine base editor with low off-target activity
Abstract
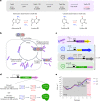
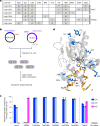
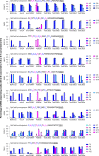
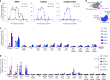
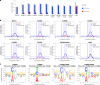
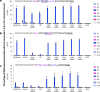
Cytosine base editors (CBEs) are larger and can suffer from higher off-target activity or lower on-target editing efficiency than current adenine base editors (ABEs). To develop a CBE that retains the small size, low off-target activity and high on-target activity of current ABEs, we evolved the highly active deoxyadenosine deaminase TadA-8e to perform cytidine deamination using phage-assisted continuous evolution. Evolved TadA cytidine deaminases contain mutations at DNA-binding residues that alter enzyme selectivity to strongly favor deoxycytidine over deoxyadenosine deamination. Compared to commonly used CBEs, TadA-derived cytosine base editors (TadCBEs) offer similar or higher on-target activity, smaller size and substantially lower Cas-independent DNA and RNA off-target editing activity. We also identified a TadA dual base editor (TadDE) that performs equally efficient cytosine and adenine base editing. TadCBEs support single or multiplexed base editing at therapeutically relevant genomic loci in primary human T cells and primary human hematopoietic stem and progenitor cells. TadCBEs expand the utility of CBEs for precision gene editing.
© 2022. The Author(s).
Conflict of interest statement
M.E.N. and D.R.L have filed patent applications on this work. D.R.L. is a consultant for Prime Medicine, Beam Therapeutics, Pairwise Plants, Chroma Medicine, Resonance Medicine and Nvelop Therapeutics, companies that use genome editing, epigenome engineering or PACE, and owns equity in these companies. M.J.O. receives compensation as a consultant for Agathos Biologics. The remaining authors declare no competing interests. Correspondence: drliu@fas.harvard.edu.
Figures
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

