Differential Effects of Chronic Methamphetamine Treatment on High-Frequency Oscillations and Responses to Acute Methamphetamine and NMDA Receptor Blockade in Conscious Mice
- PMID: 36358429
- PMCID: PMC9688055
- DOI: 10.3390/brainsci12111503
Differential Effects of Chronic Methamphetamine Treatment on High-Frequency Oscillations and Responses to Acute Methamphetamine and NMDA Receptor Blockade in Conscious Mice
Abstract
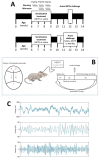
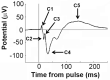
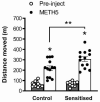
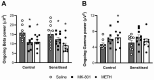
Dysregulation of high-frequency neuronal oscillations has been implicated in the pathophysiology of schizophrenia. Chronic methamphetamine (METH) use can induce psychosis similar to paranoid schizophrenia. The current study in mice aimed to determine the effect of chronic METH treatment on ongoing and evoked neuronal oscillations. C57BL/6 mice were treated with METH or vehicle control for three weeks and implanted with extradural recording electrodes. Two weeks after the last METH injection, mice underwent three EEG recording sessions to measure ongoing and auditory-evoked gamma and beta oscillatory power in response to an acute challenge with METH (2 mg/kg), the NMDA receptor antagonist MK-801 (0.3 mg/kg), or saline control. A separate group of mice pretreated with METH showed significantly greater locomotor hyperactivity to an acute METH challenge, confirming long-term sensitisation. Chronic METH did not affect ongoing or evoked gamma or beta power. Acute MK-801 challenge reduced ongoing beta power whereas acute METH challenge significantly increased ongoing gamma power. Both MK-801 and METH challenge suppressed evoked gamma power. Chronic METH treatment did not modulate these acute drug effects. There were minor effects of chronic METH and acute METH and MK-801 on selected components of event-related potential (ERP) waves. In conclusion, chronic METH treatment did not exert neuroplastic effects on the regulation of cortical gamma oscillations in a manner consistent with schizophrenia, despite causing behavioural sensitisation.
Keywords: gamma power; methamphetamine; mice; neural oscillations; sensitisation.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

Similar articles
-
Brain-derived neurotrophic factor haploinsufficiency impairs high-frequency cortical oscillations in mice.Eur J Neurosci. 2018 Oct;48(8):2816-2825. doi: 10.1111/ejn.13722. Epub 2017 Nov 6. Eur J Neurosci. 2018. PMID: 28925523
-
Effects of aberrant gamma frequency oscillations on prepulse inhibition.Int J Neuropsychopharmacol. 2014 Oct;17(10):1671-81. doi: 10.1017/S1461145714000492. Epub 2014 May 15. Int J Neuropsychopharmacol. 2014. PMID: 24832766
-
The Effect of Chronic Methamphetamine Treatment on Schizophrenia Endophenotypes in Heterozygous Reelin Mice: Implications for Schizophrenia.Biomolecules. 2020 Jun 22;10(6):940. doi: 10.3390/biom10060940. Biomolecules. 2020. PMID: 32580454 Free PMC article.
-
Cannabidiol but not cannabidiolic acid reduces behavioural sensitisation to methamphetamine in rats, at pharmacologically effective doses.Psychopharmacology (Berl). 2022 May;239(5):1593-1603. doi: 10.1007/s00213-022-06119-3. Epub 2022 Apr 18. Psychopharmacology (Berl). 2022. PMID: 35435462 Free PMC article.
-
Enhanced methamphetamine sensitisation in a rat model of the brain-derived neurotrophic factor Val66Met variant: Sex differences and dopamine receptor gene expression.Neuropharmacology. 2023 Dec 1;240:109719. doi: 10.1016/j.neuropharm.2023.109719. Epub 2023 Sep 22. Neuropharmacology. 2023. PMID: 37742717
Cited by
-
Auditory evoked-potential abnormalities in a mouse model of 22q11.2 Deletion Syndrome and their interactions with hearing impairment.Transl Psychiatry. 2025 Jan 8;15(1):4. doi: 10.1038/s41398-024-03218-x. Transl Psychiatry. 2025. PMID: 39779687 Free PMC article.
References
-
- Vearrier D., Greenberg M.I., Miller S.N., Okaneku J.T., Haggerty D.A. Methamphetamine: History, pathophysiology, adverse health effects, current trends, and hazards associated with the clandestine manufacture of methamphetamine. Dis. Mon. 2012;58:38–89. doi: 10.1016/j.disamonth.2011.09.004. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

