Neurophysiological Hallmarks of Axonal Degeneration in CIDP Patients: A Pilot Analysis
- PMID: 36358436
- PMCID: PMC9688174
- DOI: 10.3390/brainsci12111510
Neurophysiological Hallmarks of Axonal Degeneration in CIDP Patients: A Pilot Analysis
Abstract
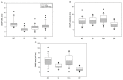
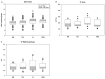
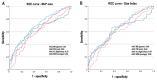
In this work, we aim to identify sensitive neurophysiological biomarkers of axonal degeneration in CIDP patients. A total of 16 CIDP patients, fulfilling the clinical and neurophysiological criteria for typical CIDP, treated with subcutaneous immunoglobulin (ScIg) (0.4 g/kg/week) were evaluated at baseline (before ScIg treatment) and after long-term treatment with ScIg (24 months) by clinical assessment scales, nerve conduction studies (NCS) and electromyography (EMG). Conventional and non-conventional neurophysiological parameters: motor unit potential (MUP) analysis, MUP thickness and size index (SI)] and interference pattern (IP) features were evaluated after long-term treatment (24 months) and compared with a population of 16 healthy controls (HC). An increase of distal motor latency (DML) and reduced compound motor action potential (CMAP) amplitude and area in CIDP patients suggest axonal damage of motor fibers, together with a significant increase of MUP amplitude, duration and area. Analysis of non-conventional MUP parameters shows no difference for MUP thickness; however, in CIDP patients, SI is increased and IP area and amplitude values are lower than HC. Despite clinical and neurophysiological improvement after ScIg treatment, neurophysiological analysis revealed axonal degeneration of motor fibers and motor unit remodeling. Correlation analysis shows that the axonal degeneration process is related to the diagnostic and therapeutic delay. MUP area and SI parameters can detect early signs of axonal degeneration, and their introduction in clinical practice may help to identify patients with the worst outcome.
Keywords: EMG; axonal degeneration; chronic inflammatory demyelinating polyneuropathy; motor unit analysis; subcutaneous immunoglobulin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Kuwabara S., Misawa S. Myelin. Springer Science & Business Media; Berlin, Germany: 2019. Chronic Inflammatory Demyelinating Polyneuropathy; pp. 333–343. - PubMed
-
- Van den Bergh P.Y.K., van Doorn P.A., Hadden R.D.M., Avau B., Vankrunkelsven P., Allen J.A., Attarian S., Blomkwist-Markens P.H., Cornblath D.R., Eftimov F., et al. European Academy of Neurology/Peripheral Nerve Society guideline on diagnosis and treatment of chronic inflammatory demyelinating polyradiculoneuropathy: Report of a joint Task Force-Second revision. J. Peripher. Nerv. Syst. 2021;26:242–268. doi: 10.1111/jns.12455. - DOI - PubMed
LinkOut - more resources
Full Text Sources

