Sodium Thiosulphate-Loaded Liposomes Control Hydrogen Sulphide Release and Retain Its Biological Properties in Hypoxia-like Environment
- PMID: 36358464
- PMCID: PMC9686859
- DOI: 10.3390/antiox11112092
Sodium Thiosulphate-Loaded Liposomes Control Hydrogen Sulphide Release and Retain Its Biological Properties in Hypoxia-like Environment
Abstract
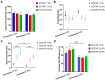
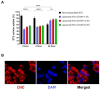
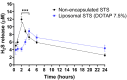
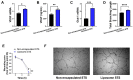
Hypoxia, or insufficient oxygen availability is a common feature in the development of a myriad of cardiovascular-related conditions including ischemic disease. Hydrogen sulphide (H2S) donors, such as sodium thiosulphate (STS), are known for their cardioprotective properties. However, H2S due to its gaseous nature, is released and cleared rapidly, limiting its potential translation to clinical settings. For the first time, we developed and characterised liposome formulations encapsulating STS and explored their potential for modulating STS uptake, H2S release and the ability to retain pro-angiogenic and biological signals in a hypoxia-like environment mirroring oxygen insufficiency in vitro. Liposomes were prepared by varying lipid ratios and characterised for size, polydispersity and charge. STS liposomal encapsulation was confirmed by HPLC-UV detection and STS uptake and H2S release was assessed in vitro. To mimic hypoxia, cobalt chloride (CoCl2) was administered in conjunction with formulated and non-formulated STS, to explore pro-angiogenic and metabolic signals. Optimised liposomal formulation observed a liposome diameter of 146.42 ± 7.34 nm, a polydispersity of 0.22 ± 0.19, and charge of 3.02 ± 1.44 mV, resulting in 25% STS encapsulation. Maximum STS uptake (76.96 ± 3.08%) from liposome encapsulated STS was determined at 24 h. Co-exposure with CoCl2 and liposome encapsulated STS resulted in increased vascular endothelial growth factor mRNA as well as protein expression, enhanced wound closure and increased capillary-like formation. Finally, liposomal STS reversed metabolic switch induced by hypoxia by enhancing mitochondrial bioenergetics. These novel findings provide evidence of a feasible controlled-delivery system for STS, thus H2S, using liposome-based nanoparticles. Likewise, data suggests that in scenarios of hypoxia, liposomal STS is a good therapeutic candidate to sustain pro-angiogenic signals and retain metabolic functions that might be impaired by limited oxygen and nutrient availability.
Keywords: angiogenesis; controlled-release; drug delivery systems; hydrogen sulphide; liposomes; mitochondrial metabolism.
Conflict of interest statement
Authors declare no conflict of interest.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources

