Microglial Hemoxygenase-1 Deletion Reduces Inflammation in the Retina of Old Mice with Tauopathy
- PMID: 36358522
- PMCID: PMC9686584
- DOI: 10.3390/antiox11112151
Microglial Hemoxygenase-1 Deletion Reduces Inflammation in the Retina of Old Mice with Tauopathy
Abstract
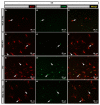
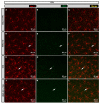
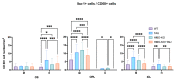
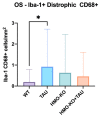
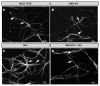
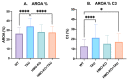
Tauopathies such as Alzheimer's disease are characterized by the accumulation of neurotoxic aggregates of tau protein. With aging and, especially, in Alzheimer's patients, the inducible enzyme heme oxygenase 1 (HO-1) progressively increases in microglia, causing iron accumulation, neuroinflammation, and neurodegeneration. The retina is an organ that can be readily accessed and can reflect changes that occur in the brain. In this context, we evaluated how the lack of microglial HO-1, using mice that do not express HO-1 in microglia (HMO-KO), impacts retinal macro and microgliosis of aged subjects (18 months old mice) subjected to tauopathy by intrahippocampal delivery of AAV-hTauP301L (TAU). Our results show that although tauopathy, measured as anti-TAUY9 and anti-AT8 positive immunostaining, was not observed in the retina of WT-TAU or HMO-KO+TAU mice, a morphometric study of retinal microglia and macroglia showed significant retinal changes in the TAU group compared to the WT group, such as: (i) increased number of activated microglia, (ii) retraction of microglial processes, (iii) increased number of CD68+ microglia, and (iv) increased retinal area occupied by GFAP (AROA) and C3 (AROC3). This retinal inflammatory profile was reduced in HMO-KO+TAU mice. Conclusion: Reduction of microglial HO-1 could be beneficial to prevent tauopathy-induced neuroinflammation.
Keywords: heme oxygenase 1 (HO-1); macroglia; microglia; neurodegenerative diseases; neuroinflammation; retina; tauopathies.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures







References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

