The Link between Oxidative Stress, Mitochondrial Dysfunction and Neuroinflammation in the Pathophysiology of Alzheimer's Disease: Therapeutic Implications and Future Perspectives
- PMID: 36358538
- PMCID: PMC9686795
- DOI: 10.3390/antiox11112167
The Link between Oxidative Stress, Mitochondrial Dysfunction and Neuroinflammation in the Pathophysiology of Alzheimer's Disease: Therapeutic Implications and Future Perspectives
Abstract
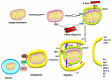
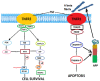
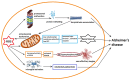
Alzheimer's disease (AD), the most common form of dementia, has increasing incidence, increasing mortality rates, and poses a huge burden on healthcare. None of the currently approved drugs for the treatment of AD influence disease progression. Many clinical trials aiming at inhibiting amyloid plaque formation, increasing amyloid beta clearance, or inhibiting neurofibrillary tangle pathology yielded inconclusive results or failed. Meanwhile, research has identified many interlinked vicious cascades implicating oxidative stress, mitochondrial dysfunction, and chronic neuroinflammation, and has pointed to novel therapeutic targets such as improving mitochondrial bioenergetics and quality control, diminishing oxidative stress, or modulating the neuroinflammatory pathways. Many novel molecules tested in vitro or in animal models have proven efficient, but their translation into clinic needs further research regarding appropriate doses, delivery routes, and possible side effects. Cell-based therapies and extracellular vesicle-mediated delivery of messenger RNAs and microRNAs seem also promising strategies allowing to target specific signaling pathways, but need further research regarding the most appropriate harvesting and culture methods as well as control of the possible tumorigenic side effects. The rapidly developing area of nanotechnology could improve drug delivery and also be used in early diagnosis.
Keywords: Alzheimer’s disease; antioxidants; mitochondrial dysfunction; nanotechnology; neuroinflammation; oxidative stress; stem cell therapies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Fratiglioni L., Launer L.J., Breteler M.M., Copeland J.R., Dartigues J.F., Lobo A., Martinez-Lage J., Soininen H., Hofman A. Incidence of dementia and major subtypes in Europe: A collaborative study of population-based cohorts. Neurologic diseases in the elderly research group. Neurology. 2000;54:1015. - PubMed
-
- Plassman B.L., Langa K.M., Fisher G.G., Heeringa S.G., Weir D.R., Ofstedal M.B., Burke J.R., Hurd M.D., Potter G.G., Rodgers W.L., et al. Prevalence of dementia in the United States: The aging, demographics, and memory study. Neuroepidemiology. 2007;29:125–132. doi: 10.1159/000109998. - DOI - PMC - PubMed
-
- Alzheimer’s Association 2019 Alzheimer’s disease facts and figures. Alzheimers Dement. 2019;15:321–387. doi: 10.1016/j.jalz.2019.01.010. - DOI
Publication types
LinkOut - more resources
Full Text Sources

