Phytochemical Analysis, Antioxidant, and Antimicrobial Activities of Ducrosia flabellifolia: A Combined Experimental and Computational Approaches
- PMID: 36358545
- PMCID: PMC9686979
- DOI: 10.3390/antiox11112174
Phytochemical Analysis, Antioxidant, and Antimicrobial Activities of Ducrosia flabellifolia: A Combined Experimental and Computational Approaches
Abstract
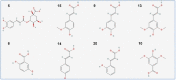
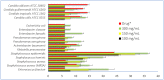
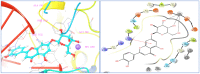
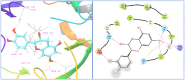
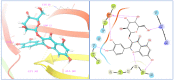
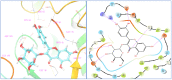
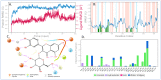
Ducrosia flabellifolia Boiss. is a rare desert plant known to be a promising source of bioactive compounds. In this paper, we report for the first time the phytochemical composition and biological activities of D. flabellifolia hydroalcoholic extract by using liquid chromatography-electrospray tandem mass spectrometry (ESI-MS/MS) technique. The results obtained showed the richness of the tested extract in phenols, tannins, and flavonoids. Twenty-three phytoconstituents were identified, represented mainly by chlorogenic acid, followed by ferulic acid, caffeic acid, and sinapic acid. The tested hydroalcoholic extract was able to inhibit the growth of all tested bacteria and yeast on agar Petri dishes at 3 mg/disc with mean growth inhibition zone ranging from 8.00 ± 0.00 mm for Enterococcus cloacae (E. cloacae) to 36.33 ± 0.58 mm for Staphylococcus epidermidis. Minimal inhibitory concentration ranged from 12.5 mg/mL to 200 mg/mL and the hydroalcoholic extract from D. flabellifolia exhibited a bacteriostatic and fungistatic character. In addition, D. flabellifolia hydroalcoholic extract possessed a good ability to scavenge different free radicals as compared to standard molecules. Molecular docking studies on the identified phyto-compounds in bacterial, fungal, and human peroxiredoxin 5 receptors were performed to corroborate the in vitro results, which revealed good binding profiles on the examined protein targets. A standard atomistic 100 ns dynamic simulation investigation was used to further evaluate the interaction stability of the promising phytocompounds, and the results showed conformational stability in the binding cavity. The obtained results highlighted the medicinal use of D. flabellifolia as source of bioactive compounds, as antioxidant, antibacterial, and antifungal agent.
Keywords: Ducrosia flabellifolia; antimicrobial; antioxidant; chemical composition; dynamic simulation; molecular docking.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Sayed-Ahmad B., Thierry T., Saad Z., Hijazi A., Merah O. The Apiaceae: Ethnomedicinal family as source for industrial uses. Ind. Crop. Prod. 2017;109:661–671. doi: 10.1016/j.indcrop.2017.09.027. - DOI
-
- Thiviya P., Gamage A., Piumali D., Merah O., Madhujith T. Apiaceae as an Important Source of Antioxidants and Their Applications. Cosmetics. 2021;8:111. doi: 10.3390/cosmetics8040111. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

