Amino Acid Solutions for 177Lu-Oxodotreotide Premedication: A Tolerance Study
- PMID: 36358631
- PMCID: PMC9657593
- DOI: 10.3390/cancers14215212
Amino Acid Solutions for 177Lu-Oxodotreotide Premedication: A Tolerance Study
Abstract
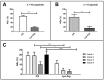
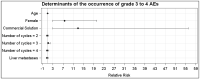
Background: The co-infusion of amino acid solutions during peptide receptor radionuclide therapy reduces the tubular reabsorption of 177Lu-oxodotreotide, thus minimizing nephrotoxicity. In our nuclear medicine department, the patients received two different types of amino acid perfusion over time: a commercial solution (CS) containing 10% amino acids, and a 2.5% lysine−arginine (LysArg) hospital preparation, produced by a referral laboratory. The aim of the present study was to analyze the tolerance of the two amino acid solutions. Methods: The patient files were analyzed and double-checked. The study parameters comprised the gender, age, primary tumor site, type of amino acid perfusion, adverse events (AE) and WHO AE grades, antiemetic premedication, creatinine, and serum potassium level. Results: From February 2016 to February 2019, 76 patients were treated, for a total 235 cycles. AEs occurred in 71% of the CS cycles (n = 82/116), versus 18% (n = 21/119) in the LysArg group (p < 0.0001). In the CS group, the AEs were mostly WHO grade 4 (n = 24/82), and mostly grade 1 in the LysArg group (n = 13/21). Poisson regression showed a higher risk of AE overall and of grades 3 and 4 in the females and with CS. The mean creatinine clearance was identical before and after the PRRT cycles, whichever amino acid perfusion was used. Conclusions: The lysine−arginine preparation showed better tolerance than the commercial solution. The change to LysArg reduced the antiemetic premedication from four molecules to one.
Keywords: 177Lu-oxodotreotide; amino acid perfusion; peptide receptor radionuclide therapy; premedication.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- De Mestier L., Lepage C., Baudin E., Coriat R., Courbon F., Couvelard A., Do Cao C., Frampas E., Gaujoux S., Gincul R., et al. Digestive Neuroendocrine Neoplasms (NEN): French Intergroup Clinical Practice Guidelines for Diagnosis, Treatment and Follow-up (SNFGE, GTE, RENATEN, TENPATH, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, SFR) Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver. 2020;52:473–492. doi: 10.1016/j.dld.2020.02.011. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

