Treatment Sequencing Strategies in Advanced Neuroendocrine Tumors: A Review
- PMID: 36358667
- PMCID: PMC9656186
- DOI: 10.3390/cancers14215248
Treatment Sequencing Strategies in Advanced Neuroendocrine Tumors: A Review
Abstract
Neuroendocrine tumor (NET) incidence has grown. The treatment landscape for advanced NETs is rapidly evolving, but there are limited head-to-head data to guide treatment sequencing decisions. We assessed the available clinical data to aid practicing clinicians in their routine clinical decision-making. Clinical trials have demonstrated efficacy benefits for new therapies in advanced NETs. Emerging long-term data from these trials have enabled clinicians to make more accurate risk-benefit assessments, particularly for patients receiving multiple lines of therapy. However, clinical data specifically regarding treatment sequencing are limited. In lieu of definitive data, treatment sequencing should be based on disease-related factors (e.g., site of tumor origin, volume of disease) and patient-related characteristics (e.g., comorbidities, patient preferences). Clinical decision-making in advanced NETs remains highly individualized and complex; important evidence gaps regarding treatment sequencing remain. Given this, advanced NET management should be a joint effort of multidisciplinary teams at referring and high-volume centers. Additional clinical trial and real-world evidence are needed to meet the challenge of understanding how to sequence available NET therapies. Until these trials are conducted, the best practices provided in this review may serve as a guide for clinicians making treatment sequencing decisions based on the available data.
Keywords: clinical trials; efficacy; neuroendocrine tumors (NET); peptide receptor radionuclide therapy (PRRT); safety; treatment sequencing.
Conflict of interest statement
Chauhan reported receiving grants from TerSera, Lexicon, Bristol Myers Squibb (BMS), Clovis Oncology, EMD Serono, Nanotherapeutics, and ECS Prograstin; and consulting fees from Novartis/Advanced Accelerator Applications, Ipsen, Lexicon, Entrensic Health Solutions, TerSera, and Seneca Therapeutics. Del Rivero declares no conflicts of interest. Li has received honoraria and advisory/consultancy fees from Lexicon, Ipsen, Eisai, Exelixis, Novartis/Advanced Accelerator Applications, Coherus, Sun Pharma, Genentech, QED, Merck, Adagene, Mina Therapeutics, Servier, Delcath, and TerSera; and received institutional research funding from Brooklyn Immunotherapeutics and AstraZeneca. Ramirez reported receiving research funding (to institution) from Merck and Aadi Bioscience; consulting fees from Amgen, Ipsen, Novartis, Advanced Accelerator Applications, Curium, EMD Serono, and AstraZeneca; and speaking fees from Ipsen and AstraZeneca. Soares has received honoraria and advisory/consultancy fees from Lexicon, Ipsen, Exelixis, Novartis/Advanced Accelerator Applications, AstraZeneca, Helsinn, Incyte, and TerSera.
Figures
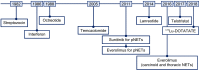

References
-
- Xu Z., Wang L., Dai S., Chen M., Li F., Sun J., Luo F. Epidemiologic trends of and factors associated with overall survival for patients with gastroenteropancreatic neuroendocrine tumors in the United States. JAMA Netw. Open. 2021;4:e2124750. doi: 10.1001/jamanetworkopen.2021.24750. - DOI - PMC - PubMed
-
- National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology for Neuroendocrine and Adrenal Tumors. 2021. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Neuroendocrine and adrenal tumors 4.2021. © National Comprehensive Cancer Network, Inc. 2022. All rights reserved. Accessed April 22, 2022. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way. [(accessed on 22 April 2022)]. Available online: https://www.NCCN.org.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

