Polygodial, a Sesquiterpene Dialdehyde, Activates Apoptotic Signaling in Castration-Resistant Prostate Cancer Cell Lines by Inducing Oxidative Stress
- PMID: 36358679
- PMCID: PMC9656647
- DOI: 10.3390/cancers14215260
Polygodial, a Sesquiterpene Dialdehyde, Activates Apoptotic Signaling in Castration-Resistant Prostate Cancer Cell Lines by Inducing Oxidative Stress
Abstract
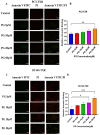
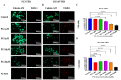
Prostate cancer (PCa) is the second leading cause of cancer death among men in the United States. Surgery, radiation therapy, chemotherapy, and androgen deprivation therapy are currently the standard treatment options for PCa. These have poor outcomes and result in the development of castration-resistant prostate cancer (CRPC), which is the foremost underlying cause of mortality associated with PCa. Taxanes, diterpene compounds approved to treat hormonal refractory PCa, show poor outcomes in CRPC. Polygodial (PG) is a natural sesquiterpene isolated from water pepper (Persicaria hydropiper), Dorrigo pepper (Tasmannia stipitata), and mountain pepper (Tasmannia lanceolata). Previous reports show that PG has an anticancer effect. Our results show that PG robustly inhibits the cell viability, colony formation, and migration of taxane-resistant CRPC cell lines and induces cell cycle arrest at the G0 phase. A toxicity investigation shows that PG is not toxic to primary human hepatocytes, 3T3-J2 fibroblast co-cultures, and non-cancerous BPH-1 cells, implicating that PG is innocuous to healthy cells. In addition, PG induces oxidative stress and activates apoptosis in drug-resistant PCa cell lines. Our mechanistic evaluation by a proteome profiler-human apoptotic array in PC3-TXR cells shows that PG induces upregulation of cytochrome c and caspase-3 and downregulation of antiapoptotic markers. Western blot analysis reveals that PG activates apoptotic and DNA damage markers in PCa cells. Our results suggest that PG exhibits its anticancer effect by promoting reactive oxygen species generation and induction of apoptosis in CRPC cells.
Keywords: anoikis; castration-resistant prostate cancer; natural products; polygodial; prostate cancer; taxane-resistant CRPC.
Conflict of interest statement
The authors declare no conflict of interest.
Figures












References
-
- American Cancer Society Key Statistics for Prostate Cancer | Prostate Cancer Facts. [(accessed on 30 April 2022)]. Available online: https://www.cancer.org/cancer/prostate-cancer/about/key-statistics.html.
LinkOut - more resources
Full Text Sources
Research Materials

