Glucose Enhances Pro-Tumorigenic Functions of Mammary Adipose-Derived Mesenchymal Stromal/Stem Cells on Breast Cancer Cell Lines
- PMID: 36358839
- PMCID: PMC9655059
- DOI: 10.3390/cancers14215421
Glucose Enhances Pro-Tumorigenic Functions of Mammary Adipose-Derived Mesenchymal Stromal/Stem Cells on Breast Cancer Cell Lines
Abstract
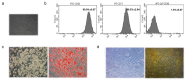
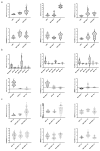
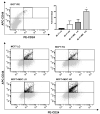
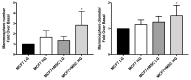
Adiposity and diabetes affect breast cancer (BC) progression. We addressed whether glucose may affect the interaction between mammary adipose tissue-derived mesenchymal stromal/stem cells (MAT-MSCs) and BC cells. Two-dimensional co-cultures and spheroids were established in 25 mM or 5.5 mM glucose (High Glucose-HG or Low Glucose-LG) by using MAT-MSCs and MCF7 or MDA-MB231 BC cells. Gene expression was measured by qPCR, while protein levels were measured by cytofluorimetry and ELISA. CD44high/CD24low BC stem-like sub-population was quantified by cytofluorimetry. An in vivo zebrafish model was assessed by injecting spheroid-derived labeled cells. MAT-MSCs co-cultured with BC cells showed an inflammatory/senescent phenotype with increased abundance of IL-6, IL-8, VEGF and p16INK4a, accompanied by altered levels of CDKN2A and LMNB1. BC cells reduced multipotency and increased fibrotic features modulating OCT4, SOX2, NANOG, αSMA and FAP in MAT-MSCs. Of note, these co-culture-mediated changes in MAT-MSCs were partially reverted in LG. Only in HG, MAT-MSCs increased CD44high/CD24low MCF7 sub-population and promoted their ability to form mammospheres. Injection in zebrafish embryos of HG spheroid-derived MCF7 and MAT-MSCs was followed by a significant cellular migration and caudal dissemination. Thus, MAT-MSCs enhance the aggressiveness of BC cells in a HG environment.
Keywords: breast cancer; glucose; mammary adipose tissue; mesenchymal stromal/stem cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Characterization of the stemness potency of mammospheres isolated from the breast cancer cell lines.Tumour Biol. 2019 Aug;41(8):1010428319869101. doi: 10.1177/1010428319869101. Tumour Biol. 2019. PMID: 31423948
-
Mammary Adipose Tissue Control of Breast Cancer Progression: Impact of Obesity and Diabetes.Front Oncol. 2020 Aug 7;10:1554. doi: 10.3389/fonc.2020.01554. eCollection 2020. Front Oncol. 2020. PMID: 32850459 Free PMC article. Review.
-
Genetic modification of human mesenchymal stem cells helps to reduce adiposity and improve glucose tolerance in an obese diabetic mouse model.Stem Cell Res Ther. 2015 Dec 9;6:242. doi: 10.1186/s13287-015-0224-9. Stem Cell Res Ther. 2015. PMID: 26652025 Free PMC article.
-
Glucose impacts onto the reciprocal reprogramming between mammary adipocytes and cancer cells.Sci Rep. 2024 Oct 21;14(1):24674. doi: 10.1038/s41598-024-76522-7. Sci Rep. 2024. PMID: 39433816 Free PMC article.
-
Mesenchymal stromal/stem cells spheroid culture effect on the therapeutic efficacy of these cells and their exosomes: A new strategy to overcome cell therapy limitations.Biomed Pharmacother. 2022 Aug;152:113211. doi: 10.1016/j.biopha.2022.113211. Epub 2022 Jun 10. Biomed Pharmacother. 2022. PMID: 35696942 Review.
Cited by
-
Human Platelet-Rich Plasma Regulates Canine Mesenchymal Stem Cell Migration through Aquaporins.Stem Cells Int. 2023 May 15;2023:8344259. doi: 10.1155/2023/8344259. eCollection 2023. Stem Cells Int. 2023. PMID: 37223543 Free PMC article.
-
Zebrafish xenografts in breast cancer research.Front Immunol. 2025 Jul 10;16:1540610. doi: 10.3389/fimmu.2025.1540610. eCollection 2025. Front Immunol. 2025. PMID: 40709187 Free PMC article. Review.
-
Targeting Resistance Pathways in Breast Cancer Through Precision Oncology: Nanotechnology and Immune Modulation Approaches.Biomedicines. 2025 Jul 10;13(7):1691. doi: 10.3390/biomedicines13071691. Biomedicines. 2025. PMID: 40722763 Free PMC article. Review.
-
The lncRNA RMST is drastically downregulated in anaplastic thyroid carcinomas where exerts a tumor suppressor activity impairing epithelial-mesenchymal transition and stemness.Cell Death Discov. 2023 Jul 1;9(1):216. doi: 10.1038/s41420-023-01514-x. Cell Death Discov. 2023. PMID: 37393309 Free PMC article.
-
Increased levels of versican and insulin-like growth factor 1 in peritumoral mammary adipose tissue are related to aggressiveness in estrogen receptor-positive breast cancer.Mol Med. 2024 Nov 5;30(1):201. doi: 10.1186/s10020-024-00968-8. Mol Med. 2024. PMID: 39501160 Free PMC article.
References
-
- Saeedi P., Petersohn I., Salpea P., Malanda B., Karuranga S., Unwin N., Colagiuri S., Guariguata L., Motala A.A., Ogurtsova K., et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res. Clin. Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

