The Monocyte, a Maestro in the Tumor Microenvironment (TME) of Breast Cancer
- PMID: 36358879
- PMCID: PMC9658645
- DOI: 10.3390/cancers14215460
The Monocyte, a Maestro in the Tumor Microenvironment (TME) of Breast Cancer
Abstract
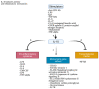
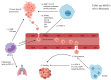
Breast cancer (BC) is well-known for being a leading cause of death worldwide. It is classified molecularly into luminal A, luminal B HER2-, luminal B HER2+, HER2+, and triple-negative breast cancer (TNBC). These subtypes differ in their prognosis; thus, understanding the tumor microenvironment (TME) makes new treatment strategies possible. The TME contains populations that exhibit anti-tumorigenic actions such as tumor-associated eosinophils. Moreover, it contains pro-tumorigenic populations such as tumor-associated neutrophils (TANs), or monocyte-derived populations. The monocyte-derived populations are tumor-associated macrophages (TAMs) and MDSCs. Thus, a monocyte can be considered a maestro within the TME. Moreover, the expansion of monocytes in the TME depends on many factors such as the BC stage, the presence of macrophage colony-stimulating factor (M-CSF), and the presence of some chemoattractants. After expansion, monocytes can differentiate into pro-inflammatory populations such as M1 macrophages or anti-inflammatory populations such as M2 macrophages according to the nature of cytokines present in the TME. Differentiation to TAMs depends on various factors such as the BC subtype, the presence of anti-inflammatory cytokines, and epigenetic factors. Furthermore, TAMs and MDSCs not only have a role in tumor progression but also are key players in metastasis. Thus, understanding the monocytes further can introduce new target therapies.
Keywords: IL-10; TNBC; breast cancer; monocyte-derived populations; monocytes; tumor microenvironment; tumor-associated macrophages.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
A cutting-edge immunomodulatory interlinkage between HOTAIR and MALAT1 in tumor-associated macrophages in breast cancer: A personalized immunotherapeutic approach.Front Mol Biosci. 2022 Oct 28;9:1032517. doi: 10.3389/fmolb.2022.1032517. eCollection 2022. Front Mol Biosci. 2022. PMID: 36387279 Free PMC article.
-
Ethnic disparities in the immune microenvironment of triple negative breast cancer and its role in therapeutic outcomes.Cancer Rep (Hoboken). 2023 Sep;6 Suppl 1(Suppl 1):e1779. doi: 10.1002/cnr2.1779. Epub 2023 Jan 12. Cancer Rep (Hoboken). 2023. PMID: 36632988 Free PMC article. Review.
-
Taraxacum mongolicum extract inhibited malignant phenotype of triple-negative breast cancer cells in tumor-associated macrophages microenvironment through suppressing IL-10 / STAT3 / PD-L1 signaling pathways.J Ethnopharmacol. 2021 Jun 28;274:113978. doi: 10.1016/j.jep.2021.113978. Epub 2021 Mar 11. J Ethnopharmacol. 2021. PMID: 33716082
-
Tumour cell derived effects on monocyte/macrophage polarization and function and modulatory potential of Viscum album lipophilic extract in vitro.BMC Complement Altern Med. 2015 Apr 24;15:130. doi: 10.1186/s12906-015-0650-3. BMC Complement Altern Med. 2015. PMID: 25902944 Free PMC article.
-
Tumor-Associated Macrophages: Protumoral Macrophages in Inflammatory Tumor Microenvironment.Adv Pharm Bull. 2020 Sep;10(4):556-565. doi: 10.34172/apb.2020.066. Epub 2020 Aug 9. Adv Pharm Bull. 2020. PMID: 33062602 Free PMC article. Review.
Cited by
-
Tumor-Associated Macrophage Targeting of Nanomedicines in Cancer Therapy.Pharmaceutics. 2023 Dec 29;16(1):61. doi: 10.3390/pharmaceutics16010061. Pharmaceutics. 2023. PMID: 38258072 Free PMC article. Review.
-
The role of tumor-associated macrophages in tumor immune evasion.J Cancer Res Clin Oncol. 2024 May 7;150(5):238. doi: 10.1007/s00432-024-05777-4. J Cancer Res Clin Oncol. 2024. PMID: 38713256 Free PMC article. Review.
-
Tumor microenvironment and drug resistance in lung adenocarcinoma: molecular mechanisms, prognostic implications, and therapeutic strategies.Discov Oncol. 2025 Feb 25;16(1):238. doi: 10.1007/s12672-025-01981-x. Discov Oncol. 2025. PMID: 40000527 Free PMC article.
-
Expression of T cell-related proteins in breast ductal carcinoma in situ.Histol Histopathol. 2025 Apr;40(4):467-475. doi: 10.14670/HH-18-805. Epub 2024 Sep 2. Histol Histopathol. 2025. PMID: 39356080
-
Secretome analysis of breast cancer cells to identify potential target proteins of Ipomoea turpethum extract-loaded nanoparticles in the tumor microenvironment.Front Cell Dev Biol. 2023 Oct 12;11:1247632. doi: 10.3389/fcell.2023.1247632. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37900279 Free PMC article.
References
-
- Zhou L., Tian Y., Guo F., Yu B., Li J., Xu H., Su Z. LincRNA-P21 Knockdown Reversed Tumor-Associated Macrophages Function by Promoting MDM2 to Antagonize* P53 Activation and Alleviate Breast Cancer Development. Cancer Immunol. Immunother. 2020;69:835–846. doi: 10.1007/s00262-020-02511-0. - DOI - PMC - PubMed
-
- Blows F.M., Driver K.E., Schmidt M.K., Broeks A., van Leeuwen F.E., Wesseling J., Cheang M.C., Gelmon K., Nielsen T.O., Blomqvist C., et al. Subtyping of Breast Cancer by Immunohistochemistry to Investigate a Relationship between Subtype and Short and Long Term Survival: A Collaborative Analysis of Data for 10,159 Cases from 12 Studies. PLoS Med. 2010;7:e1000279. doi: 10.1371/journal.pmed.1000279. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

