A Pathway Model to Understand the Evolution of Spike Protein Binding to ACE2 in SARS-CoV-2 Variants
- PMID: 36358957
- PMCID: PMC9687612
- DOI: 10.3390/biom12111607
A Pathway Model to Understand the Evolution of Spike Protein Binding to ACE2 in SARS-CoV-2 Variants
Abstract
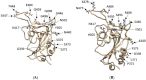
After the SARS-CoV-2 Wuhan variant that gave rise to the pandemic, other variants named Delta, Omicron, and Omicron-2 sequentially became prevalent, with mutations spread around the viral genome, including on the spike (S) protein; in order to understand the resultant in gains in infectivity, we interrogated in silico both the equilibrium binding and the binding pathway of the virus' receptor-binding domain (RBD) to the angiotensin-converting enzyme 2 (ACE2) receptor. We interrogated the molecular recognition between the RBD of different variants and ACE2 through supervised molecular dynamics (SuMD) and classic molecular dynamics (MD) simulations to address the effect of mutations on the possible S protein binding pathways. Our results indicate that compensation between binding pathway efficiency and stability of the complex exists for the Omicron BA.1 receptor binding domain, while Omicron BA.2's mutations putatively improved the dynamic recognition of the ACE2 receptor, suggesting an evolutionary advantage over the previous strains.
Keywords: Sars-Cov-2; binding pathway; molecular dynamics; spike protein ACE-2; supervised molecular dynamics.
Conflict of interest statement
J.C.M. and O.V. are employees of AstraZeneca and may hold stock in AZ.
Figures

References
-
- Gu H., Krishnan P., Ng D.Y.M., Chang L.D.J., Liu G.Y.Z., Cheng S.S.M., Hui M.M.Y., Fan M.C.Y., Wan J.H.L., Lau L.H.K., et al. Probable Transmission of SARS-CoV-2 Omicron Variant in Quarantine Hotel, Hong Kong, China, November 2021. Emerg. Infect. Dis. 2022;28:460–462. doi: 10.3201/eid2802.212422. - DOI - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

