Neuronal Firing and Glutamatergic Synapses in the Substantia Nigra Pars Reticulata of LRRK2-G2019S Mice
- PMID: 36358985
- PMCID: PMC9687271
- DOI: 10.3390/biom12111635
Neuronal Firing and Glutamatergic Synapses in the Substantia Nigra Pars Reticulata of LRRK2-G2019S Mice
Abstract
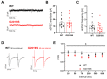
Pathogenic mutations in the leucine-rich repeat kinase 2 (LRRK2) gene are frequent causes of familial Parkinson's Disease (PD), an increasingly prevalent neurodegenerative disease that affects basal ganglia circuitry. The cellular effects of the G2019S mutation in the LRRK2 gene, the most common pathological mutation, have not been thoroughly investigated. In this study we used middle-aged mice carrying the LRRK2-G2019S mutation (G2019S mice) to identify potential alterations in the neurophysiological properties and characteristics of glutamatergic synaptic transmission in basal ganglia output neurons, i.e., substantia nigra pars reticulata (SNr) GABAergic neurons. We found that the intrinsic membrane properties and action potential properties were unaltered in G2019S mice compared to wild-type (WT) mice. The spontaneous firing frequency was similar, but we observed an increased regularity in the firing of SNr neurons recorded from G2019S mice. We examined the short-term plasticity of glutamatergic synaptic transmission, and we found an increased paired-pulse depression in G2019S mice compared to WT mice, indicating an increased probability of glutamate release in SNr neurons from G2019S mice. We measured synaptic transmission mediated by NMDA receptors and we found that the kinetics of synaptic responses mediated by these receptors were unaltered, as well as the contribution of the GluN2B subunit to these responses, in SNr neurons of G2019S mice compared to WT mice. These results demonstrate an overall maintenance of basic neurophysiological and synaptic characteristics, and subtle changes in the firing pattern and in glutamatergic synaptic transmission in basal ganglia output neurons that precede neurodegeneration of dopaminergic neurons in the LRRK2-G2019S mouse model of late-onset PD.
Keywords: LRRK2-G2019S; NMDA receptors; Parkinson’s disease; electrophysiology; glutamatergic synaptic transmission; substantia nigra reticulata.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
-
- Gonzalez-Rodriguez P., Zampese E., Surmeier D.J. Selective neuronal vulnerability in Parkinson’s disease. Prog. Brain Res. 2020;252:61–89. - PubMed
-
- Yue M., Hinkle K.M., Davies P., Trushina E., Fiesel F.C., Christenson T.A., Schroeder A.S., Zhang L., Bowles E., Behrouz B., et al. Progressive dopaminergic alterations and mitochondrial abnormalities in LRRK2 G2019S knock-in mice. Neurobiol. Dis. 2015;78:172–195. doi: 10.1016/j.nbd.2015.02.031. - DOI - PMC - PubMed
-
- Healy D.G., Falchi M., O’Sullivan S.S., Bonifati V., Durr A., Bressman S., Brice A., Aasly J., Zabetian C.P., Goldwurm S., et al. Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson’s disease: A case-control study. Lancet Neurol. 2008;7:583–590. doi: 10.1016/S1474-4422(08)70117-0. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

