YPEL3 Negatively Regulates Endometrial Function via the Wnt/β-Catenin Pathways during Early Pregnancy in Goats
- PMID: 36359097
- PMCID: PMC9656084
- DOI: 10.3390/ani12212973
YPEL3 Negatively Regulates Endometrial Function via the Wnt/β-Catenin Pathways during Early Pregnancy in Goats
Abstract
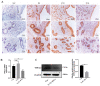
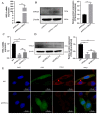
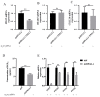
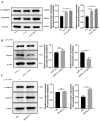
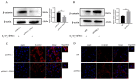
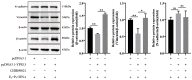
In ruminants, the establishment of pregnancy requires a series of structural and functional changes in the endometrium under the action of hormones, thereby providing an optimal environment for the implantation of the embryo. In this study, we explored the molecular mechanism by which YPEL3 regulates endometrial function during gestation in goats. We found YPEL3 expression was significantly downregulated during early gestation and that YPEL3 overexpression inhibited the expression of ISG15, but had no significant effects on the expression of RSAD2 and CXCL10 in goat endometrial epithelial cells (gEECs). In addition, YPEL3 silencing significantly inhibited PGF2α secretion and the expression of the prostaglandin synthesis-related rate-limiting enzyme-encoding genes PGFS and PTGES, with no significant effect on the expression of PTGS1 and PTGS2. Moreover, YPEL3 inhibited the expression of vimentin and β-catenin and pretreatment of gEECs with the β-catenin activator CHIR99021 prevented a YPEL3-induced decrease in vimentin expression. Collectively, our findings confirm that, as a hormone-regulated factor, YPEL3 regulates endometrial function by inhibiting the Wnt/β-catenin signaling pathway and provide new insights for further clarification of the mechanism by which YPEL3 functions during early pregnancy in ruminants.
Keywords: Wnt/β-catenin; YPEL3; endometrial function; goat endometrial epithelial cells; hormone.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

