Differential Affinity Chromatography Coupled to Mass Spectrometry: A Suitable Tool to Identify Common Binding Proteins of a Broad-Range Antimicrobial Peptide Derived from Leucinostatin
- PMID: 36359195
- PMCID: PMC9687860
- DOI: 10.3390/biomedicines10112675
Differential Affinity Chromatography Coupled to Mass Spectrometry: A Suitable Tool to Identify Common Binding Proteins of a Broad-Range Antimicrobial Peptide Derived from Leucinostatin
Abstract
Leucinostatins are antimicrobial peptides with a broad range of activities against infectious agents as well as mammalian cells. The leucinostatin-derivative peptide ZHAWOC_6027 (peptide 6027) was tested in vitro and in vivo for activity against the intracellular apicomplexan parasite Toxoplasma gondii. While highly efficacious in vitro (EC50 = 2 nM), subcutaneous application of peptide 6027 (3 mg/kg/day for 5 days) in mice experimentally infected with T. gondii oocysts exacerbated the infection, caused mild clinical signs and elevated cerebral parasite load. Peptide 6027 also impaired the proliferation and viability of mouse splenocytes, most notably LPS-stimulated B cells, in vitro. To identify common potential targets in Toxoplasma and murine splenocytes, we performed differential affinity chromatography (DAC) with cell-free extracts from T. gondii tachyzoites and mouse spleens using peptide 6027 or an ineffective analogue (peptide 21,358) coupled to N-hydroxy-succinimide sepharose, followed by mass spectrometry. Proteins specifically binding to peptide 6027 were identified in eluates from the peptide 6027 column but not in peptide 21,358 nor the mock column eluates. In T. gondii eluates, 269 proteins binding specifically to peptide 6027 were identified, while in eluates from mouse spleen extracts 645 proteins specifically binding to this peptide were detected. Both datasets contained proteins involved in mitochondrial energy metabolism and in protein processing and secretion. These results suggest that peptide 6027 interacts with common targets in eukaryotes involved in essential pathways. Since this methodology can be applied to various compounds as well as target cell lines or organs, DAC combined with mass spectrometry and proteomic analysis should be considered a smart and 3R-relevant way to identify drug targets in pathogens and hosts, thereby eliminating compounds with potential side effects before performing tedious and costly safety and efficacy assessments in animals or humans.
Keywords: animal experimentation; drug targets; mass spectrometry; modeling; peptides as drugs; side effects.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

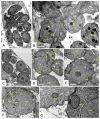
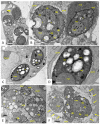







References
-
- Dubey J.P., Hemphill A., Calero-Bernal R., Schares G. Neosporosis in Animals. Volume xviii. Taylor & Francis; Boca Raton, FL, USA: 2017. 529p
-
- Dubey J.P. Toxoplasmosis of Animals and Humans. 2nd ed. CRC Press; Boca Raton, FL, USA: 2010. pp. 313–314.
-
- Müller J., Aguado-Martinez A., Balmer V., Maly D.J., Fan E., Ortega-Mora L.M., Ojo K.K., Van Voorhis W.C., Hemphill A. Two novel calcium-dependent protein kinase 1 inhibitors interfere with vertical transmission in mice infected with Neospora caninum tachyzoites. Antimicrob. Agents Chemother. 2017;61:e02324-16. doi: 10.1128/AAC.02324-16. - DOI - PMC - PubMed
-
- Basto A.P., Anghel N., Rubbiani R., Müller J., Stibal D., Giannini F., Suss-Fink G., Balmer V., Gasser G., Furrer J., et al. Targeting of the mitochondrion by dinuclear thiolato-bridged arene ruthenium complexes in cancer cells and in the apicomplexan parasite Neospora caninum. Metallomics. 2019;11:462–474. doi: 10.1039/C8MT00307F. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

