Combined DiI and Antibody Labeling Reveals Complex Dysgenesis of Hippocampal Dendritic Spines in a Mouse Model of Fragile X Syndrome
- PMID: 36359212
- PMCID: PMC9687937
- DOI: 10.3390/biomedicines10112692
Combined DiI and Antibody Labeling Reveals Complex Dysgenesis of Hippocampal Dendritic Spines in a Mouse Model of Fragile X Syndrome
Abstract
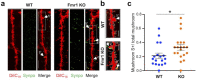
Structural, functional, and molecular alterations in excitatory spines are a common hallmark of many neurodevelopmental disorders including intellectual disability and autism. Here, we describe an optimized methodology, based on combined use of DiI and immunofluorescence, for rapid and sensitive characterization of the structure and composition of spines in native brain tissue. We successfully demonstrate the applicability of this approach by examining the properties of hippocampal spines in juvenile Fmr1 KO mice, a mouse model of Fragile X Syndrome. We find that mutant mice display pervasive dysgenesis of spines evidenced by an overabundance of both abnormally elongated thin spines and cup-shaped spines, in combination with reduced density of mushroom spines. We further find that mushroom spines expressing the actin-binding protein Synaptopodin-a marker for spine apparatus-are more prevalent in mutant mice. Previous work identified spines with Synaptopodin/spine apparatus as the locus of mGluR-LTD, which is abnormally elevated in Fmr1 KO mice. Altogether, our data suggest this enhancement may be linked to the preponderance of this subset of spines in the mutant. Overall, these findings demonstrate the sensitivity and versatility of the optimized methodology by uncovering a novel facet of spine dysgenesis in Fmr1 KO mice.
Keywords: DiIC18; Fmr1 knockout mouse; Fragile X Syndrome; dendritic spines; excitatory synapses; hippocampus; synaptopodin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Stabilization of Spine Synaptopodin by mGluR1 Is Required for mGluR-LTD.J Neurosci. 2022 Mar 2;42(9):1666-1678. doi: 10.1523/JNEUROSCI.1466-21.2022. Epub 2022 Jan 19. J Neurosci. 2022. PMID: 35046120 Free PMC article.
-
Alterations in CA1 hippocampal synapses in a mouse model of fragile X syndrome.Glia. 2018 Apr;66(4):789-800. doi: 10.1002/glia.23284. Epub 2017 Dec 23. Glia. 2018. PMID: 29274095 Free PMC article.
-
Fragile X-like behaviors and abnormal cortical dendritic spines in cytoplasmic FMR1-interacting protein 2-mutant mice.Hum Mol Genet. 2015 Apr 1;24(7):1813-23. doi: 10.1093/hmg/ddu595. Epub 2014 Nov 28. Hum Mol Genet. 2015. PMID: 25432536 Free PMC article.
-
The trouble with spines in fragile X syndrome: density, maturity and plasticity.Neuroscience. 2013 Oct 22;251:120-8. doi: 10.1016/j.neuroscience.2012.03.049. Epub 2012 Apr 20. Neuroscience. 2013. PMID: 22522472 Free PMC article. Review.
-
Dendritic spine structural anomalies in fragile-X mental retardation syndrome.Cereb Cortex. 2000 Oct;10(10):1038-44. doi: 10.1093/cercor/10.10.1038. Cereb Cortex. 2000. PMID: 11007554 Review.
Cited by
-
Mouse models of fragile X-related disorders.Dis Model Mech. 2023 Feb 1;16(2):dmm049485. doi: 10.1242/dmm.049485. Epub 2023 Jan 24. Dis Model Mech. 2023. PMID: 36692473 Free PMC article. Review.
-
LUZP1 Regulates Dendritic Spine Maturation and Synaptic Plasticity in the Hippocampal Dentate Gyrus of Mice.J Neurosci. 2025 May 14;45(20):e1867242025. doi: 10.1523/JNEUROSCI.1867-24.2025. J Neurosci. 2025. PMID: 40180573
-
Synaptopodin: a key regulator of Hebbian plasticity.Front Cell Neurosci. 2024 Nov 6;18:1482844. doi: 10.3389/fncel.2024.1482844. eCollection 2024. Front Cell Neurosci. 2024. PMID: 39569068 Free PMC article. Review.
-
From wings to whiskers to stem cells: why every model matters in fragile X syndrome research.J Neurodev Disord. 2024 Jun 13;16(1):30. doi: 10.1186/s11689-024-09545-w. J Neurodev Disord. 2024. PMID: 38872088 Free PMC article. Review.
-
Loss of synaptopodin impairs mGluR5 and protein synthesis-dependent mGluR-LTD at CA3-CA1 synapses.PNAS Nexus. 2024 Feb 8;3(2):pgae062. doi: 10.1093/pnasnexus/pgae062. eCollection 2024 Feb. PNAS Nexus. 2024. PMID: 38384385 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

