Novel Classification of Thrombotic Disorders Based on Molecular Hemostasis and Thrombogenesis Producing Primary and Secondary Phenotypes of Thrombosis
- PMID: 36359229
- PMCID: PMC9687744
- DOI: 10.3390/biomedicines10112706
Novel Classification of Thrombotic Disorders Based on Molecular Hemostasis and Thrombogenesis Producing Primary and Secondary Phenotypes of Thrombosis
Abstract
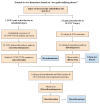
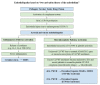
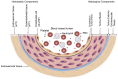
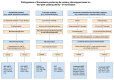
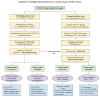
Thrombosis, the common and deadliest disorder among human diseases, develops as a result of the intravascular hemostasis following an intravascular injury, which can be caused by a variety of trauma, non-traumatic insults or clinical illnesses. Thrombosis can occur at any location of the vascular system supplied by blood from the heart to large and smallest arterial and venous systems and may affect the function and anatomy of the organ and tissue. It more commonly occurs in the smaller circulatory system of the vascular tree such as arterioles and capillaries, and venules of the organs, especially in the brain, lungs, heart, pancreas, muscle and kidneys, and sinusoids of the liver. Thrombosis has been referred as the disease of "blood clots", which concept is incompletely defined, but represents many different hemostatic diseases from microthrombosis to fibrin clot disease, macrothrombosis, and combined micro-macrothrombosis. Thrombosis is produced following an intravascular injury via one or more combination of four different mechanisms of thrombogenesis: microthrombogenesis, fibrinogenesis, macrothrombogenesis and micro-macrothrombogenesis initiated by normal physiological hemostasis in vivo. The clinical phenotype expression of thrombosis is determined by: (1) depth of the intravascular wall injury, (2) extent of the injury affecting the vascular tree system, (3) physiological character of the involved vascular system, (4) locality of the vascular injury, and (5) underlying non-hemostatic conditions interacting with hemostasis. Recent acquisition of "two-path unifying theory" of hemostasis and "two-activation theory of the endothelium" has opened a new frontier in science of medicine by identifying the pathophysiological mechanism of different thrombotic disorders and also contributing to the better understanding of many poorly defined human diseases, including different phenotypes of stroke and cardiovascular disease, trauma, sepsis and septic shock, multiorgan dysfunction syndrome, and autoimmune disease, and others. Reviewed are the fundamentals in hemostasis, thrombogenesis and thrombosis based on hemostatic theories, and proposed is a novel classification of thrombotic disorders.
Keywords: combined micro-macrothrombosis; endotheliopathy; fibrin clot disease; fibrinogenesis; hemostasis; macrothrombogenesis; macrothrombosis; microthrombogenesis; microthrombosis; thrombogenesis; thrombosis; vascular microthrombotic disease.
Conflict of interest statement
The author Jae C. Chang has neither financial nor non-financial competing interest in regard to this article.
Figures
Similar articles
-
Pathogenesis of Two Faces of DVT: New Identity of Venous Thromboembolism as Combined Micro-Macrothrombosis via Unifying Mechanism Based on "Two-Path Unifying Theory" of Hemostasis and "Two-Activation Theory of the Endothelium".Life (Basel). 2022 Jan 31;12(2):220. doi: 10.3390/life12020220. Life (Basel). 2022. PMID: 35207507 Free PMC article. Review.
-
Disseminated intravascular coagulation: new identity as endotheliopathy-associated vascular microthrombotic disease based on in vivo hemostasis and endothelial molecular pathogenesis.Thromb J. 2020 Oct 14;18:25. doi: 10.1186/s12959-020-00231-0. eCollection 2020. Thromb J. 2020. PMID: 33061857 Free PMC article. Review.
-
Thrombogenesis and thrombotic disorders based on 'two-path unifying theory of hemostasis': philosophical, physiological, and phenotypical interpretation.Blood Coagul Fibrinolysis. 2018 Nov;29(7):585-595. doi: 10.1097/MBC.0000000000000769. Blood Coagul Fibrinolysis. 2018. PMID: 30234545 Review.
-
Molecular Pathogenesis of Endotheliopathy and Endotheliopathic Syndromes, Leading to Inflammation and Microthrombosis, and Various Hemostatic Clinical Phenotypes Based on "Two-Activation Theory of the Endothelium" and "Two-Path Unifying Theory" of Hemostasis.Medicina (Kaunas). 2022 Sep 19;58(9):1311. doi: 10.3390/medicina58091311. Medicina (Kaunas). 2022. PMID: 36143988 Free PMC article. Review.
-
Vaccine-Associated Thrombocytopenia and Thrombosis: Venous Endotheliopathy Leading to Venous Combined Micro-Macrothrombosis.Medicina (Kaunas). 2021 Oct 26;57(11):1163. doi: 10.3390/medicina57111163. Medicina (Kaunas). 2021. PMID: 34833382 Free PMC article. Review.
Cited by
-
Exploring metabolite-mediated links between lipidome and deep vein thrombosis: Insights from Mendelian randomization analysis.Medicine (Baltimore). 2025 Mar 7;104(10):e41783. doi: 10.1097/MD.0000000000041783. Medicine (Baltimore). 2025. PMID: 40068057 Free PMC article.
-
Post-translational modifications of fibrinogen: implications for clotting, fibrin structure and degradation.Mol Biomed. 2024 Oct 31;5(1):45. doi: 10.1186/s43556-024-00214-x. Mol Biomed. 2024. PMID: 39477884 Free PMC article. Review.
-
Exploring the Mechanism of Chuanxiong Rhizoma against Thrombosis Based on Network Pharmacology, Molecular Docking and Experimental Verification.Molecules. 2023 Sep 19;28(18):6702. doi: 10.3390/molecules28186702. Molecules. 2023. PMID: 37764479 Free PMC article.
-
Retrospective analysis of venous thromboembolism, arterial thromboembolism, and microthrombosis incidence at a single center during the COVID-19 pandemic.Medicine (Baltimore). 2024 Oct 11;103(41):e39915. doi: 10.1097/MD.0000000000039915. Medicine (Baltimore). 2024. PMID: 39465786 Free PMC article.
-
A Systematic Review of Endothelial Dysfunction in Chronic Venous Disease-Inflammation, Oxidative Stress, and Shear Stress.Int J Mol Sci. 2025 Apr 12;26(8):3660. doi: 10.3390/ijms26083660. Int J Mol Sci. 2025. PMID: 40332237 Free PMC article.
References
-
- Chang J.C. Thrombocytopenia in critically ill patients due to vascular microthrombotic disease: Pathogenesis based on “two activation theory of the endothelium”. Vascul. Dis. Ther. 2017;2:1–7. doi: 10.15761/VDT.1000132. - DOI

