The Evolving Scenario in the Assessment of Radiological Response for Hepatocellular Carcinoma in the Era of Immunotherapy: Strengths and Weaknesses of Surrogate Endpoints
- PMID: 36359347
- PMCID: PMC9687474
- DOI: 10.3390/biomedicines10112827
The Evolving Scenario in the Assessment of Radiological Response for Hepatocellular Carcinoma in the Era of Immunotherapy: Strengths and Weaknesses of Surrogate Endpoints
Abstract
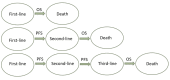
Hepatocellular carcinoma (HCC) is a challenging malignancy characterised by clinical and biological heterogeneity, independent of the stage. Despite the application of surveillance programs, a substantial proportion of patients are diagnosed at advanced stages when curative treatments are no longer available. The landscape of systemic therapies has been rapidly growing over the last decade, and the advent of immune-checkpoint inhibitors (ICIs) has changed the paradigm of systemic treatments. The coexistence of the tumour with underlying cirrhosis exposes patients with HCC to competing events related to tumour progression and/or hepatic decompensation. Therefore, it is relevant to adopt proper clinical endpoints to assess the extent of treatment benefit. While overall survival (OS) is the most accepted endpoint for phase III randomised controlled trials (RCTs) and drug approval, it is affected by many limitations. To overcome these limits, several clinical and radiological outcomes have been used. For instance, progression-free survival (PFS) is a useful endpoint to evaluate the benefit of sequential treatments, since it is not influenced by post-progression treatments, unlike OS. Moreover, radiological endpoints such as time to progression (TTP) and objective response rate (ORR) are frequently adopted. Nevertheless, the surrogacy between these endpoints and OS in the setting of unresectable HCC (uHCC) remains uncertain. Since most of the surrogate endpoints are radiology-based (e.g., PFS, TTP, ORR), the use of standardised tools is crucial for the evaluation of radiological response. The optimal way to assess the radiological response has been widely debated, and many criteria have been proposed over the years. Furthermore, none of the criteria have been validated for immunotherapy in advanced HCC. The coexistence of the underlying chronic liver disease and the access to several lines of treatments highlight the urgent need to capture early clinical benefit and the need for standardised radiological criteria to assess cancer response when using ICIs in mono- or combination therapies. Here, we review the most commonly used clinical and radiological endpoints for trial design, as well as their surrogacy with OS. We also review the criteria for radiological response to treatments for HCC, analysing the major issues and the potential future perspectives.
Keywords: HCC; RECIST 1.1; endpoints; hepatocellular carcinoma; immunotherapy; mRECIST; radiological criteria; systemic therapy.
Conflict of interest statement
R.C: support for attending meetings from Bracco and Bayer; co-funding by the European Union-FESR or FSE, PON Research and Innovation 2014–2020—DM 1062/2021.
Figures


References
-
- Vitale A., Svegliati-Baroni G., Ortolani A., Cucco M., Dalla Riva G.V., Giannini E.G., Piscaglia F., Rapaccini G., Di Marco M., Caturelli E., et al. Epidemiological trends and trajectories of MAFLD-associated hepatocellular carcinoma 2002–2033: The ITA.LI.CA database. Gut. 2021 doi: 10.1136/gutjnl-2021-324915. - DOI - PubMed
-
- Cabibbo G., Petta S., Barbàra M., Missale G., Virdone R., Caturelli E., Piscaglia F., Morisco F., Colecchia A., Farinati F., et al. A meta-analysis of single HCV-untreated arm of studies evaluating outcomes after curative treatments of HCV-related hepatocellular carcinoma. Liver Int. 2017;37:1157–1166. doi: 10.1111/liv.13357. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

