Graphene-Based Sensor for the Detection of Cortisol for Stress Level Monitoring and Diagnostics
- PMID: 36359436
- PMCID: PMC9689560
- DOI: 10.3390/diagnostics12112593
Graphene-Based Sensor for the Detection of Cortisol for Stress Level Monitoring and Diagnostics
Abstract
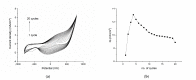
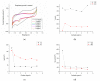
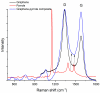
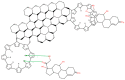
In this work, we study the sensing properties of multi-layer graphene combined with pyrrole in order to elaborate low-cost, high-sensitive material for cortisol detection. Graphene nanoplatelets and pyrrole were dispersed in a solution containing 1M HNO3 by using a powerful ultrasound probe for 10 min, then centrifuged for 30 min at 4000 rpm; polymerization was performed by cyclic voltammetry. The graphene-pyrrole composite was tested to ultra-low levels of cortisol in artificial saliva, consistent to the levels excreted in human salivary samples. The composite was further investigated by Raman spectroscopy and we modeled the interaction between the sensitive layer and cortisol using MarvinBeans software. It shows a good sensitivity for salivary values of cortisol cyclic voltammetry being able to detect a level down to 0.5 ng/mL cortisol.
Keywords: cortisol; cyclic voltammetry; electrochemical sensors; graphene-based sensing materials; graphene/pyrrole.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Chai J., Zhang J., Wen Y., Zou L., Zhang X., Xin X., Zhou M., Xu J., Zhang G. Highly sensitive electrochemical sensor based on pedot: Pss-β-cd-swcnt-cooh moded glassy carbon electrode enables trace analysis shikonin. J. Electrochem. Soc. 2019;166:B388. doi: 10.1149/2.0471906jes. - DOI
-
- Atta N.F., El-Ads E.H., Galal A., Galal A.E. Electrochemical sensing platform based on nano-perovskite/glycine/carbon composite for amlodipine and ascorbic acid drugs. Electroanalysis. 2019;31:448–460. doi: 10.1002/elan.201800577. - DOI
-
- Mead M., Popoola O., Stewart G., Landsho P., Calleja M., Hayes M., Baldovi J., McLeod M., Hodgson T., Dicks J., et al. The use of electrochemical sensors for monitoring urban air quality in low-cost, high-density networks. Atmos. Environ. 2013;70:186–203. doi: 10.1016/j.atmosenv.2012.11.060. - DOI
Grants and funding
- 16N/08.02.2019/Unitatea Executiva Pentru Finantarea Invatamantului Superior a Cercetarii Dezvoltarii si Inovarii
- PN 19 06 01 01/2019./Unitatea Executiva Pentru Finantarea Invatamantului Superior a Cercetarii Dezvoltarii si Inovarii
- PN-III-P1-1.1-PD-2019-1134 (Ctr. No. PD87/2020)/Unitatea Executiva Pentru Finantarea Invatamantului Superior a Cercetarii Dezvoltarii si Inovarii
- PN-III-P2-2.1-PED-2019-2551/Unitatea Executiva Pentru Finantarea Invatamantului Superior a Cercetarii Dezvoltarii si Inovarii
- 18PFE/30.12.2021/Unitatea Executiva Pentru Finantarea Invatamantului Superior a Cercetarii Dezvoltarii si Inovarii
LinkOut - more resources
Full Text Sources

