Approach of Acromegaly during Pregnancy
- PMID: 36359512
- PMCID: PMC9689290
- DOI: 10.3390/diagnostics12112669
Approach of Acromegaly during Pregnancy
Abstract
Acromegaly-related sub/infertility, tidily related to suboptimal disease control (1/2 of cases), correlates with hyperprolactinemia (1/3 of patients), hypogonadotropic hypogonadism—mostly affecting the pituitary axis in hypopituitarism (10−80%), and negative effects of glucose profile (GP) anomalies (10−70%); thus, pregnancy is an exceptional event. Placental GH (Growth Hormone) increases from weeks 5−15 with a peak at week 37, stimulating liver IGF1 and inhibiting pituitary GH secreted by normal hypophysis, not by somatotropinoma. However, estrogens induce a GH resistance status, protecting the fetus form GH excess; thus a full-term, healthy pregnancy may be possible. This is a narrative review of acromegaly that approaches cardio-metabolic features (CMFs), somatotropinoma expansion (STE), management adjustment (MNA) and maternal-fetal outcomes (MFOs) during pregnancy. Based on our method (original, in extenso, English—published articles on PubMed, between January 2012 and September 2022), we identified 24 original papers—13 studies (3 to 141 acromegalic pregnancies per study), and 11 single cases reports (a total of 344 pregnancies and an additional prior unpublished report). With respect to maternal acromegaly, pregnancies are spontaneous or due to therapy for infertility (clomiphene, gonadotropins or GnRH) and, lately, assisted reproduction techniques (ARTs); there are no consistent data on pregnancies with paternal acromegaly. CMFs are the most important complications (7.7−50%), especially concerning worsening of HBP (including pre/eclampsia) and GP anomalies, including gestational diabetes mellitus (DM); the best predictor is the level of disease control at conception (IGF1), and, probably, family history of 2DM, and body mass index. STE occurs rarely (a rate of 0 to 9%); some of it symptoms are headache and visual field anomalies; it is treated with somatostatin analogues (SSAs) or alternatively dopamine agonists (DAs); lately, second trimester selective hypophysectomy has been used less, since pharmaco-therapy (PT) has proven safe. MNA: PT that, theoretically, needs to be stopped before conception—continued if there was STE or an inoperable tumor (no clear period of exposure, preferably, only first trimester). Most data are on octreotide > lanreotide, followed by DAs and pegvisomant, and there are none on pasireotide. Further follow-up is required: a prompt postpartum re-assessment of the mother’s disease; we only have a few data confirming the safety of SSAs during lactation and long-term normal growth and developmental of the newborn (a maximum of 15 years). MFO seem similar between PT + ve and PT − ve, regardless of PT duration; the additional risk is actually due to CMF. One study showed a 2-year median between hypophysectomy and pregnancy. Conclusion: Close surveillance of disease burden is required, particularly, concerning CMF; a personalized approach is useful; the level of statistical evidence is expected to expand due to recent progress in MNA and ART.
Keywords: acromegaly; cabergoline; diabetes mellitus; growth hormone; lanreotide; octreotide; pegvisomant; pituitary; pregnancy; prolactin; somatostatin; somatotropinoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
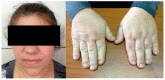
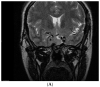

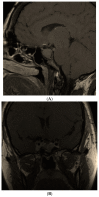

References
-
- Dal J., Feldt-Rasmussen U., Andersen M., Kristensen L.Ø., Laurberg P., Pedersen L., Dekkers O.M., Sørensen H.T., Jørgensen J.O. Acromegaly incidence, prevalence, complications and long-term prognosis: A nationwide cohort study. Eur. J. Endocrinol. 2016;175:181–190. doi: 10.1530/EJE-16-0117. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

