Nogo-A Regulates the Fate of Human Dental Pulp Stem Cells toward Osteogenic, Adipogenic, and Neurogenic Differentiation
- PMID: 36359811
- PMCID: PMC9657129
- DOI: 10.3390/cells11213415
Nogo-A Regulates the Fate of Human Dental Pulp Stem Cells toward Osteogenic, Adipogenic, and Neurogenic Differentiation
Abstract
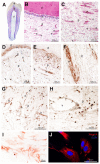
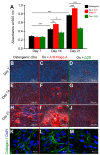
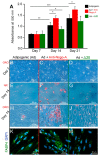
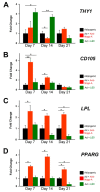
Human teeth are highly innervated organs that contain a variety of mesenchymal stem cell populations that could be used for cell-based regenerative therapies. Specific molecules are often used in these treatments to favorably modulate the function and fate of stem cells. Nogo-A, a key regulator of neuronal growth and differentiation, is already used in clinical tissue regeneration trials. While the functions of Nogo-A in neuronal tissues are extensively explored, its role in teeth still remains unknown. In this work, we first immunohistochemically analyzed the distribution of Nogo-A protein in the dental pulp of human teeth. Nogo-A is localized in a variety of cellular and structural components of the dental pulp, including odontoblasts, fibroblasts, neurons and vessels. We also cross-examined Nogo expression in the various pulp cell clusters in a single cell RNA sequencing dataset of human dental pulp, which showed high levels of expression in all cell clusters, including that of stem cells. We then assessed the role of Nogo-A on the fate of human dental pulp stem cells and their differentiation capacity in vitro. Using immunostaining, Alizarin Red S, Nile Red and Oil Red O staining we showed that Nogo-A delayed the differentiation of cultured dental pulp stem cells toward the osteogenic, adipogenic and neurogenic lineages, while addition of the blocking anti-Nogo-A antibody had opposite effects. These results were further confirmed by qRT-PCR, which demonstrated overexpression of genes involved in osteogenic (RUNX2, ALP, SP7/OSX), adipogenic (PPAR-γ2, LPL) and neurogenic (DCX, TUBB3, NEFL) differentiation in the presence of the anti-Nogo-A antibody. Conversely, the osteogenic and adipogenic genes were downregulated by Nogo-A. Taken together, our results show that the functions of Nogo-A are not restricted to neuronal cells but are extended to other cell populations, including dental pulp stem cells. We show that Nogo-A regulates their fates toward osteogenic, adipogenic and neurogenic differentiation, thus indicating its potential use in clinics.
Keywords: Nogo-A; adipogenic potential; anti-Nogo-A antibody; dental pulp; differentiation; human; human dental pulp stem cells; in vitro; neurogenic potential; osteogenic potential; single cell RNA sequencing; stem cell fates; tooth.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Limited Adipogenic Differentiation Potential of Human Dental Pulp Stem Cells Compared to Human Bone Marrow Stem Cells.Int J Mol Sci. 2024 Oct 16;25(20):11105. doi: 10.3390/ijms252011105. Int J Mol Sci. 2024. PMID: 39456888 Free PMC article.
-
A comparative in vitro study of the osteogenic and adipogenic potential of human dental pulp stem cells, gingival fibroblasts and foreskin fibroblasts.Sci Rep. 2019 Feb 11;9(1):1761. doi: 10.1038/s41598-018-37981-x. Sci Rep. 2019. PMID: 30741963 Free PMC article.
-
A nanoscale ridge/groove pattern arrayed surface enhances adipogenic differentiation of human supernumerary tooth-derived dental pulp stem cells in vitro.Arch Oral Biol. 2014 Aug;59(8):765-74. doi: 10.1016/j.archoralbio.2014.04.014. Epub 2014 May 2. Arch Oral Biol. 2014. PMID: 24837475
-
Stem cells of the dental pulp.Indian J Dent Res. 2012 Jul-Aug;23(4):558. doi: 10.4103/0970-9290.104977. Indian J Dent Res. 2012. PMID: 23257502 Review.
-
The Wisdom in Teeth: Neuronal Differentiation of Dental Pulp Cells.Cell Reprogram. 2023 Feb;25(1):32-44. doi: 10.1089/cell.2022.0102. Epub 2023 Jan 31. Cell Reprogram. 2023. PMID: 36719998 Free PMC article. Review.
Cited by
-
Mesenchymal stem cells under epigenetic control - the role of epigenetic machinery in fate decision and functional properties.Cell Death Dis. 2023 Nov 6;14(11):720. doi: 10.1038/s41419-023-06239-4. Cell Death Dis. 2023. PMID: 37932257 Free PMC article. Review.
-
Metformin-mediated effects on mesenchymal stem cells and mechanisms: proliferation, differentiation and aging.Front Pharmacol. 2024 Aug 13;15:1465697. doi: 10.3389/fphar.2024.1465697. eCollection 2024. Front Pharmacol. 2024. PMID: 39193338 Free PMC article. Review.
-
An unexpected role of neurite outgrowth inhibitor A as regulator of tooth enamel formation.Int J Oral Sci. 2024 Oct 20;16(1):60. doi: 10.1038/s41368-024-00323-x. Int J Oral Sci. 2024. PMID: 39426966 Free PMC article.
-
Limited Adipogenic Differentiation Potential of Human Dental Pulp Stem Cells Compared to Human Bone Marrow Stem Cells.Int J Mol Sci. 2024 Oct 16;25(20):11105. doi: 10.3390/ijms252011105. Int J Mol Sci. 2024. PMID: 39456888 Free PMC article.
-
Mutanobactin-D, a Streptococcus mutans Non-Ribosomal Cyclic Lipopeptide, Induces Osteogenic/Odontogenic Differentiation of Human Dental Pulp Stem Cells and Human Bone Marrow Stem Cells.Int J Mol Sci. 2025 Jan 28;26(3):1144. doi: 10.3390/ijms26031144. Int J Mol Sci. 2025. PMID: 39940912 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

