The Hidden Role of Non-Canonical Amyloid β Isoforms in Alzheimer's Disease
- PMID: 36359817
- PMCID: PMC9654995
- DOI: 10.3390/cells11213421
The Hidden Role of Non-Canonical Amyloid β Isoforms in Alzheimer's Disease
Abstract
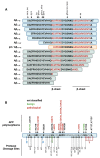
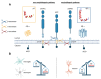
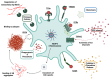
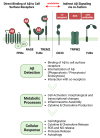
Recent advances have placed the pro-inflammatory activity of amyloid β (Aβ) on microglia cells as the focus of research on Alzheimer's Disease (AD). Researchers are confronted with an astonishing spectrum of over 100 different Aβ variants with variable length and chemical modifications. With the exception of Aβ1-42 and Aβ1-40, the biological significance of most peptides for AD is as yet insufficiently understood. We therefore aim to provide a comprehensive overview of the contributions of these neglected Aβ variants to microglia activation. First, the impact of Aβ receptors, signaling cascades, scavenger mechanisms, and genetic variations on the physiological responses towards various Aβ species is described. Furthermore, we discuss the importance of different types of amyloid precursor protein processing for the generation of these Aβ variants in microglia, astrocytes, oligodendrocytes, and neurons, and highlight how alterations in secondary structures and oligomerization affect Aβ neurotoxicity. In sum, the data indicate that gene polymorphisms in Aβ-driven signaling pathways in combination with the production and activity of different Aβ variants might be crucial factors for the initiation and progression of different forms of AD. A deeper assessment of their interplay with glial cells may pave the way towards novel therapeutic strategies for individualized medicine.
Keywords: APP; Alzheimer’s Disease; Amyloid Beta; cell-surface receptors; glia; microglia; neurodegeneration; neuroinflammation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

