Phenotypical and Functional Alteration of γδ T Lymphocytes in COVID-19 Patients: Reversal by Statins
- PMID: 36359845
- PMCID: PMC9656060
- DOI: 10.3390/cells11213449
Phenotypical and Functional Alteration of γδ T Lymphocytes in COVID-19 Patients: Reversal by Statins
Abstract
(1) Background: statins have been considered an attractive class of drugs in the pharmacological setting of COVID-19 due to their pleiotropic properties and their use correlates with decreased mortality in hospitalized COVID-19 patients. Furthermore, it is well known that statins, which block the mevalonate pathway, affect γδ T lymphocyte activation. As γδ T cells participate in the inflammatory process of COVID-19, we have investigated the therapeutical potential of statins as a tool to inhibit γδ T cell pro-inflammatory activities; (2) Methods: we harvested peripheral blood mononuclear cells (PBMCs) from COVID-19 patients with mild clinical manifestations, COVID-19 recovered patients, and healthy controls. We performed ex vivo flow cytometry analysis to study γδ T cell frequency, phenotype, and exhaustion status. PBMCs were treated with Atorvastatin followed by non-specific and specific stimulation, to evaluate the expression of pro-inflammatory cytokines; (3) Results: COVID-19 patients had a lower frequency of circulating Vδ2+ T lymphocytes but showed a pronounced pro-inflammatory profile, which was inhibited by in vitro treatment with statins; (4) Conclusions: the in vitro capacity of statins to inhibit Vδ2+ T lymphocytes in COVID-19 patients highlights a new potential biological function of these drugs and supports their therapeutical use in these patients.
Keywords: COVID-19; SARS-CoV-2 infection; mevalonate pathway; statin; γδ T cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


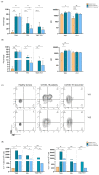
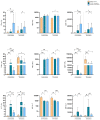
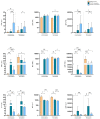
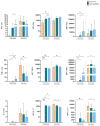

References
-
- Gupta A., Madhavan M.V., Poterucha T.J., DeFilippis E.M., Hennessey J.A., Redfors B., Eckhardt C., Bikdeli B., Platt J., Nalbandian A., et al. Association Between Antecedent Statin Use and Decreased Mortality in Hospitalized Patients with COVID-19. Res. Sq. 2021;12:1–9. doi: 10.1038/s41467-021-21553-1. - DOI - PMC - PubMed
-
- Ridker P.M., Danielson E., Fonseca F.A., Genest J., Gotto A.M., Jr., Kastelein J.J., Koenig W., Libby P., Lorenzatti A.J., MacFadyen J.G., et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N. Engl. J. Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

