Precision Phenotyping of Nectar-Related Traits Using X-ray Micro Computed Tomography
- PMID: 36359848
- PMCID: PMC9658521
- DOI: 10.3390/cells11213452
Precision Phenotyping of Nectar-Related Traits Using X-ray Micro Computed Tomography
Abstract
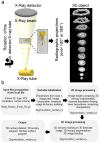
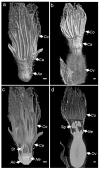
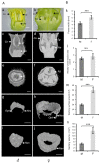
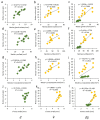
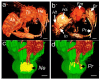
Flower morphologies shape the accessibility to nectar and pollen, two major traits that determine plant-pollinator interactions and reproductive success. Melon is an economically important crop whose reproduction is completely pollinator-dependent and, as such, is a valuable model for studying crop-ecological functions. High-resolution imaging techniques, such as micro-computed tomography (micro-CT), have recently become popular for phenotyping in plant science. Here, we implemented micro-CT to study floral morphology and honey bees in the context of nectar-related traits without a sample preparation to improve the phenotyping precision and quality. We generated high-quality 3D models of melon male and female flowers and compared the geometric measures. Micro-CT allowed for a relatively easy and rapid generation of 3D volumetric data on nectar, nectary, flower, and honey bee body sizes. A comparative analysis of male and female flowers showed a strong positive correlation between the nectar gland volume and the volume of the secreted nectar. We modeled the nectar level inside the flower and reconstructed a 3D model of the accessibility by honey bees. By combining data on flower morphology, the honey bee size and nectar volume, this protocol can be used to assess the flower accessibility to pollinators in a high resolution, and can readily carry out genotypes comparative analysis to identify nectar-pollination-related traits.
Keywords: Cucumis melo; X-ray microtomography (micro-CT); flower; honey bee; image analysis; nectar; nectary; pollinators.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Dorado J., Vázquez D.P. The diversity-stability relationship in floral production. Oikos. 2014;123:1137–1143. doi: 10.1111/oik.00983. - DOI
-
- Kiill L.H.P., Feitoza E.D.A., Siqueira K.M.M.D., Ribeiro M.D.F., Silva E.M.S.D. Evaluation of Floral Characteristics of Melon Hybrids (Cucumis Melo L.) in Pollinator Attractiveness. Rev. Bras. Frutic. 2016;38:1–12. doi: 10.1590/0100-29452016531. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

